Reports: ND956112-ND9: Fabrication and Modeling of a Novel Class of Porous Media
Muhammad Sahimi, PhD, University of Southern California
We proposed a novel fabrication method called SPONGE – structured porous network generation. In a nutshell, SPONGE is as follows. A salt (e.g. NaCl), suspended in a non-soluble medium, e.g. an alcohol or ketone, is used to coat a nonporous surface, such as plastic films, metal foils, or a glass. The salt consists of cubic crystals and, therefore, represents a granular porous medium of non-spherical particles with a distribution of particle sizes. A thermoplastic polymeric film, e.g. polyolefin, or polyvinyl chloride (vinyl) is then hot-pressed over the salt layer. The polymeric material fills the void space between the salt’s crystals, and solidifies upon cooling. The salt layer is then washed off by water. The voids created by removing the salt crystals create and expose the porous medium. In principle, any other powder other than salt can be used, provided that it can be washed off by a proper solvent after the polymeric matrix has solidified. But, for the same of discussion simplicity we refer to all such powders as “salt.” have succeeded.
SPONGE has many distinct advantages over practically all the previous methods: (i) since the porous medium is prepared by invading the salt packing, washing it off is easy, as all the crystals are accessible through their contact with each other. (ii) The pore-size distribution and pore connectivity of the porous sample are controlled completely by the size distribution of the salt crystals, their shapes, and their packing. The voids that are created by washing off the salt are the pores through which fluid flow and transport, as well as sorption and reaction occur. Thus, the size distribution of the pores is exactly the same as that of the salt crystals, which can be measured before the porous medium is even fabricated. Thus, one has complete information on the pore space morphology, and no longer needs to use such methods as the BET and mercury porosimetry in order to determine the pore-size distribution. (iii) One can also design any size distribution by selecting the appropriate crystal shapes and size distribution. Thus, for fabricating porous media to purify the used water produced in the oil industry, the particle size will be selected in such a way that it can capture a significant amount of the impurities and particles that are suspended in the water. (iv) The size of the pores may also be varied in a controlled way. If, for example, we add a small amount of a nonvolatile liquid (such as propylene glycol, glycerin, etc.), or a water-soluble polymer to the solvent, then, upon drying, the added liquid or water-soluble polymer will make capillary bridges in the contact area between the particles and expand the size of the pores. After imbibition by the molten polymer and its solidification, the salt and the nonvolatile liquid, or the water-soluble polymer, are leached out, leaving behind the larger pores. Larger and longer pores may also be created in the pore space, if the solution is mixed with soluble fibers, or rod-like crystals. After the fibers are washed off, they leave behind large pores. (v) Since the salt crystals form a packing of particles in which all the particles are in contact, there can be no isolated porosity in the final porous media. In fact, due to the packing structure otherwise, the packing will not be mechanically stable. Thus, practically all the pore space is accessible, and there is virtually no dead-end porosity either.
We have succeeded in fabricating the first generation of porous materials by SPONGE. A paper reporting on the method and the results have been submitted.
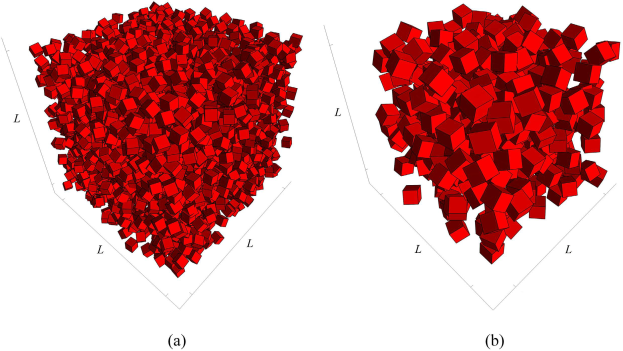
To understand better the properties of the fabricated porous materials, we have also been carrying out extensive and large-scale computer simulation of packing of cubic particles that are used in the fabrication of the porous media. Surprisingly, before our work, no serious attempt had been made to study the properties of such packings, even though they have many potential and realized applications.
Our computer simulations have progressed very significantly. We have proposed for the first time an efficient algorithm for constructing a packing of cubic particles, and have used it to study the morphological properties of such packings. Both monodisperse and polydisperse packings have been studied, and three papers have been published, reporting the first results of our simulations. Examples of such packings are presented below
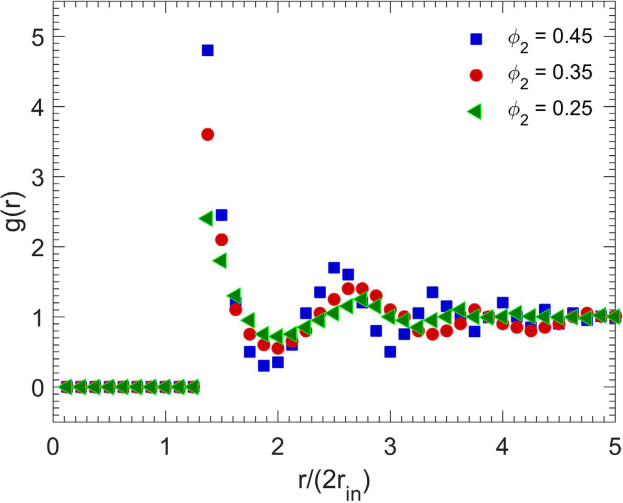
Various properties of such packings have also been computed and reported. For example, the radial distribution function g(r) and its dependence on the particle density are shown below.