Reports: ND456315-ND4: Organic Chemistry Studies in Solution via Single-Molecule Fluorescence Microscopy
Suzanne A. Blum, University of California, Irvine
Goals
Single-molecule fluorescence microscopy techniques have emerged in the last nine years as powerful tools for studying organic chemistry and catalysis on surfaces—but not in freely diffusing solution phases. Many of these examples, while groundbreaking, have limited relevance to current mechanistic questions in solution-phase organic chemistry. Motivated by the importance of chemistry that occurs in the solution phase, we herein aim to develop and demonstrate a new technique for imaging the chemistry of freely diffusing molecules using single-molecule fluorescence microscopy. This technique will provide insight into chemical reaction mechanisms by avoiding the ensemble averaging that obscures certain reactivity information in traditional measurements.
Progress Report
Move of collaborator: 1.5-year slow-down on instrument access. Our collaborator, Prof. Dr. Thorben Cordes, moved his laboratory from the University of Groningen to the Ludwig Maximilian University of Munich (LMU). This process has resulted in dismantling and rebuilding of the home-built fluorescence microscopy laser system that we intended to use together for part of this collaboration. Thus, we were unable to initiate collaborative experiments on this part of the project on the timeline expected. This instrument has 3D scanning skills that our in-house/-lab microscope does not, making finding interfaces in solution possible. The instrument is expected to be rebuilt at its new location in 2018. The PI, Prof. Blum, is applying for renewed Humboldt sabbatical support to visit LMU in Spring 2019 to assist in these experiments. For this reason, we anticipate asking for a no cost time extension for the grant funding in the future.
Alternative approaches. Due to the unavailability of the expected equipment, we have pursued the following three alternative approaches:
1) Use of viscous solvents to slow diffusion, coupled with use of our in-house microscope and a microscope capable of single-particle tracking in a shared-use facility, the UCI Laboratory for Fluorescence Dynamics (LFD). We investigated paraffin and other viscous hydrocarbons to restrict diffusion, especially to restrict molecular diffusion along the z-axis. For these studies, imaging agent 1 was doped into an active catalytic system with a ruthenium ring-opening-polymerization catalyst (Grubbs II) and untagged norbornene. The ratio of doping was ~1:106, such that individual insertion reactions of 1 could be observed in the growing polymers (eq 1).
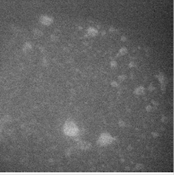
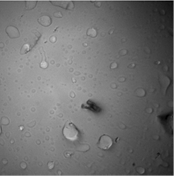
Our goal was to slow diffusion such that individual insertion reactions of 1 could be detected in solution-phase polymer growth. Figure 1 shows representative data from our in-house microscope that summarizes the results of this approach by postdoctoral scholar Dr. Nozomi Saito. The main challenge with this approach was that either the polymers could be observed affixed to the surface of the microscope slide (Figure 1a and 1b), rather than freely diffusing in solution as desired; or, the polymers could be observed slowly diffusing but they were too large, bright, and diffuse to detect single molecule chemistry (Figure 1c). For these reasons, this approach was ultimately not successful.
Figure 1. (a) Polymers affixed to the glass microscope slide viewed by fluorescence, in which individual chemical reactions can be observed, (b) same polymers, now viewed in epi mode, showing bright background and fixed locations of polymers, and (c) solution-phase imaging of this sample shows that diffuse polymers slowly moving above the glass can be observed by fluorescence, however, individual chemical reactions are not detected.
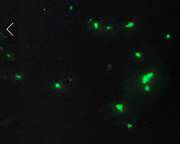
2) Use of catalysts dispersed in a gel in order to slow diffusion. As PI, Prof. Blum was in the laboratory during summer 2017 herself and performed gel dispersion experiments in order to assist the project. Probe 1 was indeed incorporated into the gel, but appeared to continue to move sufficiently such that its signal was only detectible as a bright haze rather than as individual bright point sources of light consistent with single-molecule imaging. Figure 2 shows a representative image under these conditions. This approach was ultimately not successful for that reason.
Figure 2. Bright green particles are ~1-4 mm pieces of PDMS that have been treated to disperse Grubbs II catalyst, followed by treatment with imaging agent 1. The green color shows that the PDMS has incorporated 1, possibly through ROMP reactions, however, individual molecules of 1 are not resolved.
3) We then discovered that polymerization in large, dynamic polymer systems moved, but slowly enough for detection of single insertion events within the growing polymers with our in-house microscope. These systems are considerably more fluid than the systems that have been able to be studied by single-molecule microscopy previously, and further, contain molecular catalysts without a requirement to tether them to glass surfaces. This approach is thus successful. Additional experiments using this approach are underway. These experiments are being conducted by postdoctoral scholar Dr. Nozomi Saito. Submission of a manuscript for publication is expected in 2018.
Plan for next project period. As mentioned, we anticipate requesting a no cost time extension on account of the laboratory move by the collaborator, in order to complete the cross-coupling solution-phase experiments. In addition, we will complete the ongoing studies on the large, dynamic polymer systems (as identified in Alternative Approach 3).