Reports: ND956324-ND9: Understanding Asphaltene Dispersion through Coupled Surface Activity and Rheological Studies
Chinedum Osuji, PhD, Yale University
PRF 2017 Annual Report: 56324-ND9: 09/2016-08/2017
Executive Summary
This project concerns the development of improved methods for describing the dispersion of colloidal particles in suspensions using surfactants (dispersants). YEAR 1 efforts focused on training a new student in the execution of the required surface activity measurements and rheological characterizations. Additional effort was directed towards analyzing data on an analogous system, based on suspension of carbon black in non-polar fluids. These efforts resulted in robust identification of surface activity parameters and a strong correlation with the rheology of suspensions prepared using the studied dispersants.
Research Narrative
Introduction: Maintaining suspension stability by effective particle dispersion in systems with attractive interactions can be accomplished by the addition of dispersants that modify the inter-particle potential to provide steric or electrostatic barriers against aggregation. The efficacy of such dispersants is typically considered simply by the modification of suspension rheological properties as a function of the overall added dispersant concentration. Such considerations however do little to reveal the molecular origin of differences in dispersant efficacy as they do not consider differences in surface activity. Our goal is to improve the means by which we understand dispersant efficacy in suspensions of carbonaceous materials, and in particular, in asphaltenes. Asphaltenes are high molar mass species with significant polar and polyaromatic character that often feature alkyl side chains and heteroatoms such as O, N, S, Ni and V. Asphaltenes can precipitate readily from crudes and deposit on the walls of pipelines as the system undergoes changes of temperature, pressure and composition during extraction and transport. Importantly, asphaltenes also play a significant role in fouling by promoting the flocculation and deposition of other heavy species such as diamondoids, asphaltogenic acids and resins. Here, we used data on carbon black suspensions to validate the proposed approach, which entails a combination of surface activity and rheological data to describe dispersant efficacy in a manner that is more relevant to molecular understanding than currently done.
We combine measured adsorption isotherms with rheological characterization of the elasticity of colloidal gels formed by particle aggregation to provide a more meaningful assessment of dispersant efficacy. The rheological data show the dispersants are effective in reducing particle aggregation while, from the adsorption isotherms, they differ considerably in their surface coverage at constant overall concentrations. When compared at constant dispersant particle surface coverage, the gel rheology shows marked differences across the different dispersants, as opposed to comparisons at constant overall dispersant concentration in the suspensions. In particular, the power-law volume fraction scaling of gel elasticity at constant coverage reveals clear differences in the critical volume fraction for gel formation for the different dispersants. The most efficacious dispersant is that associated with the largest critical volume fraction for gel formation at given surface coverage. Our results thus far demonstrate the utility of rheological investigations coupled with accurate determinations of surface coverage to better differentiate dispersant performance, which may improve efforts to engineer new dispersant molecules. A manuscript was published: Langmuir 2018, 34 (3), pp 1092–1099 (DOI: 10.1021/acs.langmuir.7b03343). Ongoing efforts are now extending the developed methodology to asphaltenes. More detailed summaries of experimental results and analysis of experimental data are provided below.
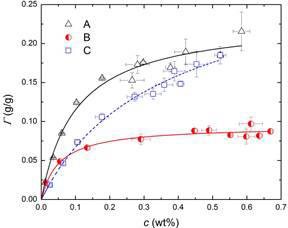
Results: Adsorption isotherms were measured by mass-balance usnig FTIR measurements on filtered solutions of carbon black particles. Samples were prepared by separation of carbon black from suspensions containing different quantities of 3 dispersants (here denoted “A”, “B” and “C”) using a 0.2 µm filter. The adsorption isotherms are plotted in Figure 1. The data are well-described by the Langmuir model (Equation 1) where G and Gmax are the amount of surface-adsorbed dispersant (i.e. the surface coverage) and its maximum value, respectively, K is an equilibrium constant that reflects the binding affinity, and c is the equilibrium concentration of the dispersant in solution (i.e. free dispersant). Equation 2 provides the mass balance that relates the overall dispersant concentration (ct) to the free dispersant concentration c, the carbon black content, cCB, and the coverage, G. Table 1 shows the results of fitting to the Langmuir isotherm.
c/ G = (c/ Gmax )+1/( GmaxK)(1)
ct=[1-(G+1)cCB]c+GcCB(2)
Figure 1. Langmuir adsorption isotherms. The lines show the best fit of the experimental data points using Equation 1.
Table 1. Fitting parameters of Equation 1 for Langmuir adsorption isotherms shown in Figure 1.
Dispersant | Γmax(g/g) | K |
A | 0.240 | 9.15 |
B | 0.094 | 19.90 |
C | 0.290 | 3.09 |
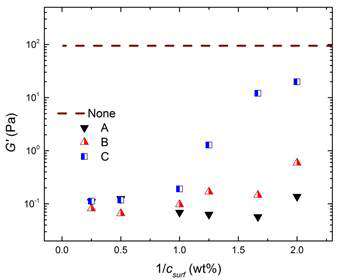
Rheological measurements were performed to investigate the stabilizing effect of the dispersants on the suspensions microstructure. Following the common approach, linear viscoelastic (LVE) properties of a 4 wt.% carbon black suspension in oil were evaluated at various dispersant concentrations. The results for storage modulus at 10 rad/s are demonstrated in Figure 2 as a function of the inverse of overall dispersant concentration used to formulate the samples.
Figure 2: Effect of dispersant mass concentration on elastic modulus (at ω = 10 rad/s) in a 4 wt.% suspension. Note that the x-axis is the inverse of the dispersant concentration. The dashed line shows the results without any dispersants.
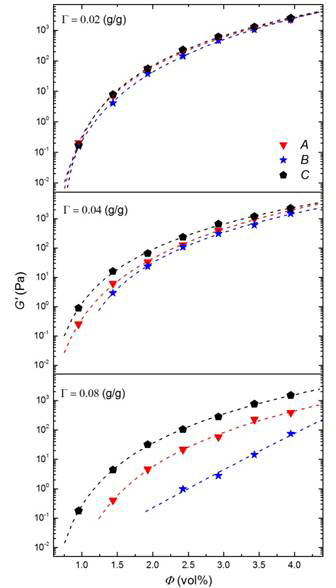
Using the adsorption isotherms in Figure 1 and Langmuir correlation, samples of 4 wt.% carbon black with sufficient quantities of each dispersant were prepared to obtain similar surface coverages for each of the dispersants. A small quantity of each sample was then filtered and the exact amounts of the free concentration and coverage were determined to ensure the prepared suspensions at a certain coverage overlap on the adsorption isotherms within the experimental error. The effect of dispersant on the linear viscoelastic properties of a 4 wt.% carbon black suspension was considered at three surface coverages of 0.02 (g/g), 0.04 (g/g) and 0.08 (g/g). Rheological studies conducted on these suspensions showed a clear delineation in the dispersant efficacy at constant covereage as parameterized by the critical volume fraction for gelation (Figure 3)
Figure 3. Scaling behavior of frequency-independent elastic modulus with carbon black volume concentration for different dispersants at various coverages. The lines demonstrate the best fit of the experimental data to extract critical gelation compositions.