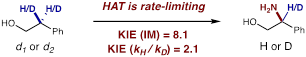Reports: DNI155704-DNI1: Catalyst-Directed C-H Functionalization for the Streamlined Use of Petroleum Feedstocks
David A. Nagib, PhD, Ohio State University
Excellent progress has been made toward our goal of developing new methods to elaborate petroleum feedstock chemicals via targeted C-H functionalization. For example, in a recently published article, we described the development of a new protocol for the β C-H amination of unactivated, secondary C-H bonds to form a broad range of β-amino alcohols from feedstock alcohols. To successfully achieve this goal, we developed a new radical-chaperone activation strategy, in which selective hydrogen atom transfer (HAT)-mediated abstraction of unbiased C-H bonds could be achieved via in situ generation and sequestration of transiently generated N-centered imidate radicals. This approach combined our triiodide-mediated activation strategy (PRF Year 1) with a novel method for in situ generation of imidate radicals (PRF Year 2), which enabled the first HAT-based mechanism of imidates. Notably, this strategy also represents the first, and only, way of directly accessing N(sp2) radicals from imidates. By harnessing the radical reactivity of these easily accessed (alcohol-derived) imidates, we demonstrated the utility of a conceptually new strategy for directed C-H amination as a way to access a range of biologically relevant β-amino alcohols from petroleum-derived feedstocks.
Progress
Below is an outline of the progress that we have made towards each of the specific aims outlined for this project.
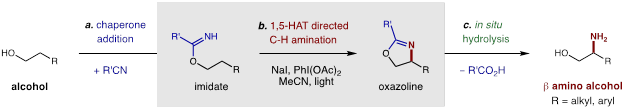
Directed β C-H Amination of Alcohols via Radical Chaperones.
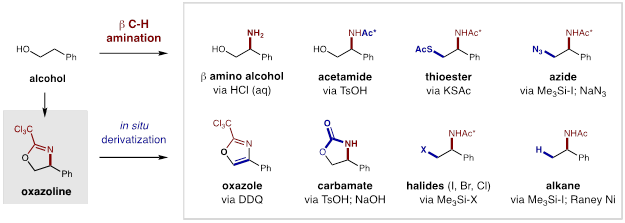
In order to realize our goal of developing broadly applicable synthetic methods for targeted C-H functionalization of petroleum feedstock chemicals, we strove to develop the classic Hofmann-Löffler-Freytag (HLF) reaction. Building on its enabling potential, we first addressed a major limitation in which the C-H bonds must be biased or activated (e.g. tertiary, benzylic, α-oxy). In Year 1, we were able to extend the synthetic scope of this remote amination to include unbiased, secondary C-H bonds. In Year 2, we then turned our attention to executing our proposal of enabling specific control of the C-H functionalization event through the design and development of a radical chaperone. Ultimately, we discovered that imidates (easily prepared by condensation of alcohols with nitriles) are excellent radical precursors that allow for in situ radical generation and subsequent C-H amination. Following a canonical 1,5-HAT mechanism, these three atom tethers enable directed β C-H amination. Upon selective formation of an oxazoline, an in situ hydrolysis affords ready access to pharmacologically relevant β-amino alcohols. Notably, by further developing our triiodide-based strategy, we were able to extend this amination to a range of unactivated alkyl C-H bonds. Interestingly, this three-stage protocol allows for rapid conversion of an alcohol to its β-amino alcohol analog in a single afternoon.
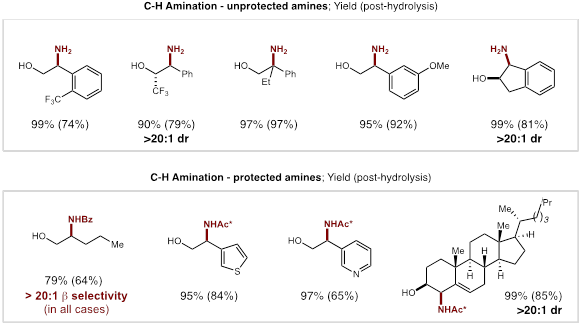
Through an acid/base work-up and extractive purification, we also demonstrated that the quantitative C-H amination products could be obtained in high yields (>75%) with excellent regioselectivity (>20:1), and without column chromatography. In the cases of secondary alcohols, excellent diastereoselectivity (>20:1) were also observed. The applicability of this strategy to the streamlined derivatization of medicines was also illustrated by the β C-H amination of the imidates of cholesterol and ibuprofen. An abbreviated substrate table is included below, which showcases the wide scope and utility of this reaction for access to both protected and unprotected β-amino alcohols by C-H functionalization of their alcohol-derived precursors.
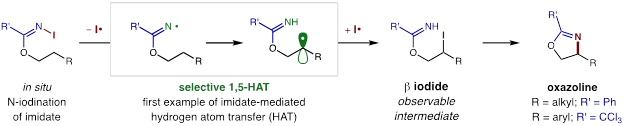
Employing our triiodide-based solution for remote amination of secondary C-H bonds, we propose a selective 1,5-HAT mechanism ensues from the visible-light-homolysis of in situ generated N-iodo imidates. The subsequent C-H functionalization reaction represents the first example of an imidate-mediated HAT, and affords the first access to a β-iodo alcohol via a radical mechanism. This intermediate rapidly undergoes intramolecular cyclization to afford the isolated oxazolines – directly from the free N-H imidate – in a single transformation.
Our preliminary mechanistic experiments indicate that the key HAT step is rate-limiting in this reaction. This conclusion is supported by KIE measurements of >8. Meanwhile, further mechanistic experiments suggest the intramolecular amination step likely determines the stereochemical outcome of the highly diastereoselective amination.
Interestingly, the immediate product of the β C-H amination is an oxazoline heterocycle that offers rapid access to a wide range of other, useful amines. In addition to acidic hydrolysis to access either the protected or unprotected β-amino alcohols, further manipulations enable one-step access to a variety of β-substituted amines via nucleophilic substitution of the alcohol-derived C-O bond. Several useful examples of nucleophilic derivations that we demonstrated include synthesis of thioesters, azides, and halides, which can be subsequently reduced to access the branched alkyl amines. Most notably, subsequent oxidation of this heterocycle to an oxazole, is also a powerful demonstration of the utility of this C-H functionalization technology to combine an alcohol and a nitrile to rapidly access these valuable heteroarenes.
Impact
The successful completion and publication of this ACS PRF-funded research has had a major impact on the establishment of our research lab as well as the effective training of personnel who were supported by this grant, including three undergraduate students, five graduate students, and one postdoctoral fellow. Our dissemination of this research has garnered positive attention in the synthetic organic and C-H functionalization communities, through fruitful interactions at numerous conferences (regional, national, and international) and invited seminars around the world. It has also served as a seed for several ongoing collaborations to investigate the mechanism and medicinal applications of these methods. Moreover, each of these seminal publications from our lab has been highlighted individually by the journal, Synform, including on the cover of one of its issues. Additional highlights of these C-H aminations by the Organic Chemistry Portal and the Org. Process Res. Dev. also illustrate the impact and broad utility of these strategies. Recently, we also published a review, which provides comprehensive coverage of the history, utility, and ongoing challenges of the field of C-H functionalization via HAT. Importantly, based on preliminary results obtained through this PRF grant, our lab was awarded an R35 MIRA from the National Institutes of Health (NIH), as well as the CAREER Award from the National Science Foundation (NSF), and the 2017 Thieme Chemistry Journal Award.