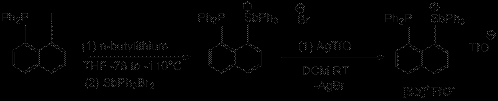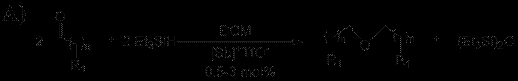Reports: UR354584-UR3: Antimony(V) Cations as Lewis Acid Catalysts for C-F Activation
Todd Hudnall, PhD, Texas State University
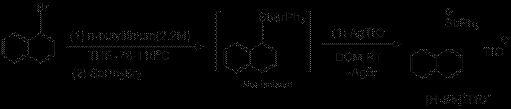
Scheme 1: Synthesis of 1-diphenylphosphinonaphthyl-8-triphenylstibonium triflate ([Sb]+TfO-) and 1- triphenylstiboniumnaphthyl triflate ([H-Sb]+TfO-).
1.2 Catalytic Applications of [Sb]+TfO- and [H-Sb]+TfO-: Depsite the lack of reactivity with small molecules, we found that both [Sb]+TfO- and [H-Sb]+TfO- serve as potent Lewis acid catalysts for a variety of transformations including the reductive coupling of aldehydes, self Aldol condensation and cyclcotrimerization of aldehydes (Scheme 2). These reactions were shown to take place at low catalyst loadings (0.5—3 mol%) and proceeded in good to excellent yields. Moreover, there is some selectivity observed between the two antimony cations, however these results are very new and we haven’t clearly determined what those specific differences are.
Scheme 2: A) selective reductive coupling, B) self Aldol condensation, and C) cyclotrimerization of aldehydes catalyzed by antimony(V) cations ([Sb]+)
{n = 2, 8, R1 = Me; n = 0, 2, R1 = Ph; n = 0; R1 = (Me)2CH, (Et)2CH, Cyclohexyl, (Ph)2CH, C6F5, (orto-Br)Ph, (m-Br)Ph, (m-F)Ph, (para-CF3)Ph, (para-NO2)Ph, and (para-Me)Ph} Yield > 90%
(R1 = Me, n =2, 8; R1 = Ph, n = 1, 2) Yield > 90% n = 2, 8, R1 = Me; n = 1, 2, R1 = Ph; n = 0, R1 = (Et2)CH, Cy, and (Ph)2CH}
Impressively, the cycloctrimerization reaction shown in Scheme 2C does not require the use of any solvent, and therefore we believe this to be a novel green synthesis of 1,3,5 trioxanes. In addition, the antimony(V) cations can catalyze the conversion of aldehydes to acetals in the presence of triethoxysilane (Scheme 3) without the need for any organic solvent.
Scheme 3: Solvent free conversion of aldehydes and (EtO)3SiH into acetals catalyzed by [Sb]+
1.3 Outcomes of Research: We prepared three manuscripts for publication, 2 are published, 1 is currently under review:
Ugarte, R. A.; Devarajan, D.; Mushinski, R. M. and Hudnall, T. W.* “Antimony(V) Cations for the Selective Catalytic Transformation of Aldehydes Into Symmetric Ethers, a,b-Unsaturated Aldehydes, and 1,3,5-Trioxanes” Dalton Trans. 2016, 45, 11150-11161.
Ugarte, R. A.; Hudnall, T. W.* “Antimony(V) Catalyzed Acetalisation of Aldehydes: An Efficient, Solvent-Free, and Recyclable Green Process” Green Chem. 2017, 19, 1990-1998.
Melancon, K. M.; Gildner, M. B.; Hudnall, T. W.* “Synthesis, Spectroscopic Characterization, and Redox Reactivity of a CAAC-Derived Arsamethine Cyanine Dye” Angew. Chem. Int. Ed. 2018, submitted..