Reports: DNI754951-DNI7: Reaction Kinetic Elucidation and Gas Transport Characterization of Interfacially Photopolymerized, Intrinsically Microporous Membranes
Timothy F. Scott, PhD, University of Michigan
This project involved the improvement of membrane-based gas separation efficiency, a challenge that remains a formidable owing to the compromise between attaining high selectivity and high permeability. The reaction kinetics of interfacial copper (I)-catalyzed azide–alkyne cycloaddition (CuAAC) photopolymerizations using rigid monomeric precursors and the structure and gas separation characteristics of the resultant (micro)porous organic polymer thin films were initially examined; however, owing to the irreversibility of the CuAAC reaction, the cross-linked, (micro)porous organic polymer (MOP) films generated by reaction of tetrakis(4-ethynylphenyl)methane with azide-bearing monomers were found to be amorphous with varying pore dimensions. Thus, covalent organic framework (COF) polymers, crystalline cross-linked materials fabricated using dynamic covalent bond-forming reactions, were synthesized and characterized using monomers analogous to those used for the MOP films.
Synthesis of Imine-Linked 3D COFs
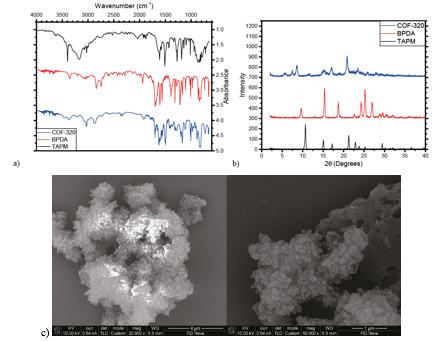
COF-300 was successfully synthesized by solvothermolysis from tetrakis(4-aminophenyl)methane (TAPM) and terephthalaldehyde (TPA) in 1,4-dioxane and acetic acid at 120°C for 72 hours. FTIR spectroscopy confirmed the disappearance of the amine and aldehyde peaks and the appearance of the characteristic imine stretch (Fig. 1a). Powder X-ray diffraction (PXRD) results show a crystalline structure, with diffraction peaks in agreement with previously published results (Fig. 1b); in addition, no diffraction peaks from starting materials were observed in the PXRD spectrum of COF-300 (Fig. 1c). SEM images showed a clear crystal structure (Fig. 1d). The surface area of COF-300 was determined by N2 adsorption/desorption isotherm to be 425 m2 g-1.
COF-320 was similarly synthesized by solvothermolysis from TAPM and 4-4'-biphenyldialdehyde (BPDA). FTIR confirmed the disappearance of the amine and aldehyde peaks and the appearance of imine stretch peaks (Fig. 2a). PXRD results showed a crystalline structure, with a slightly raised baseline indicating the presence of some amorphous materials; no diffraction peaks from starting materials were observed in the PXRD spectrum of COF-320 (Fig. 2b). SEM images revealed rice-shaped crystals (Fig. 2c), confirming the PXRD results.
Figure 1. Characterization of COF-300. a) FTIR spectra of COF-300 compared to starting materials TAPM and TPA; b) comparison of PXRD spectra between literature (top) and experimental (bottom); c) comparison of COF-300 PXRD spectrum to starting materials TAPM and terephthalaldehyde; and d) SEM images of COF-300, in which crystal structures can clearly be observed.
Synthesis of Functionalized COF
Modification of the basic structure of a three-dimensional COF is challenging. To enable structural modification for a broader range of applications, we aim to functionalize the aldehyde precursor to include a reactive group that does not affect with the imine formation and exchange reactions.
Figure 2. Characterization of COF-320. a) FTIR spectra of COF-320 compared to starting materials TAPM and BPDA; b) comparison of COF-320 PXRD spectrum to starting materials TAPM and 4,4'-biphenyldialdehyde; and c) SEM images of COF-320, in which a mixture of crystal and amorphous structures can be observed.
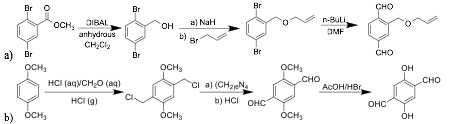
The first reaction pathway designed, shown in Fig. 3a, converted methyl-2,5-dibromobenzoate into an allyloxy-functionalized terephthalaldehyde. We successfully conducted the first two steps of the synthesis, obtaining intermediates in high purity and reasonable yields. The complications arose in the last step owing to n-butyllithium once again. Further investigation into the conversion of various dibromobenzene derivatives into corresponding terephthalaldehydes showed that once one bromine atom is replaced by an aldehyde group, the second substitution does not take place.
A subsequent synthetic pathway designed to synthesize a functionalized terephthalaldehyde, shown in Fig. 3b, eschews the usage of n-butyllithium entirely, instead relying on a wholly different reaction mechanism to convert methyl chloride groups into aldehyde groups.
Sc(OTf)3 Catalyzed COF-300 Synthesis
Figure 3. Synthesis pathways for a) functionalized dialdehyde linker 2-((allyloxy)methyl)terephthal-aldehyde; and b) functionalized dialdehyde linker 2,5-dihydroxyterephthalaldehyde (DHTA).
Traditionally, imine-based COFs are synthesized by solvothermolysis at 120°C for 72 hours, with acetic acid as the catalyst. Mechanistic studies of the imine-linked COF formation process have revealed that amines and aldehydes rapidly precipitate into an amorphous polymer network, which slowly rearranges into a crystalline structure during the extended heating period by imine exchange reactions. Given the rate-limiting nature of these reactions, identifying a more effective catalyst than acetic acid for transimination is paramount. Lewis acidic metal triflates are highly active for mediating both imine formation and transimination, and possess excellent water tolerance and functional group compatibility, making them strong candidates for COF synthesis. Our attempts to synthesize COF-300 utilizing Sc(OTf)3 yielded rapid formation of yellow amorphous precipitate (confirmed by PXRD) was observed at room temperature using catalyst loadings of as low as 0.01 equivalents. After 72 hours, no change was observed in their PXRD spectra.
In-situ Deprotection of TPA in COF-300 Synthesis
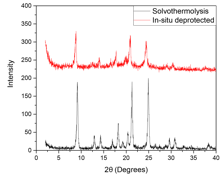
Figure 4. PXRD spectra of COF-300 synthesized from solvothermolysis (black) versus Sc(OTf)3-mediated in-situ deprotection (red).
To overcome the kinetic trapping limitations, we need to limit the rate of formation of the amorphous polymer network at the start of the reaction. An acetal protecting group was introduced to protect TPA prior to synthesis, and can be removed in-situ by Sc(OTf)3. In this reaction regime, there is a distinct lack of aldehyde functional groups in the reaction mixture at the start of the reaction, and the imine formation reaction is rate-limited by the rate of aldehyde deprotection. Here, Sc(OTf)3 served as a dual-role catalyst for both the deprotection of the aldehyde groups and the imine formation and transimination reactions. Optimization of reaction conditions is currently in progress, but we have successfully obtained crystalline structures comparable to those obtained through the solvothermolysis method, as shown in Fig. 4.