Reports: UR754033-UR7: Development of Supramolecular Polymers Based on Novel Methylene-Bridge-Linked Multi-Calixarenes
Jordan L. Fantini, PhD, Denison University
Two aspects of the project were pursued during this reporting period. First, we explored the binding properties of model systems with a single host molecule (H) and a ditopic guest (G––G). Second, we began the synthetic route to a similar A—B monomer with the potential to use environmental parameters to effect polymerization/depolymerization.
The system studied was the sulfonated calixarene with a ditopic guest based on the DABCO unit. The length of the linker between the units was varied between one and six methylene groups.
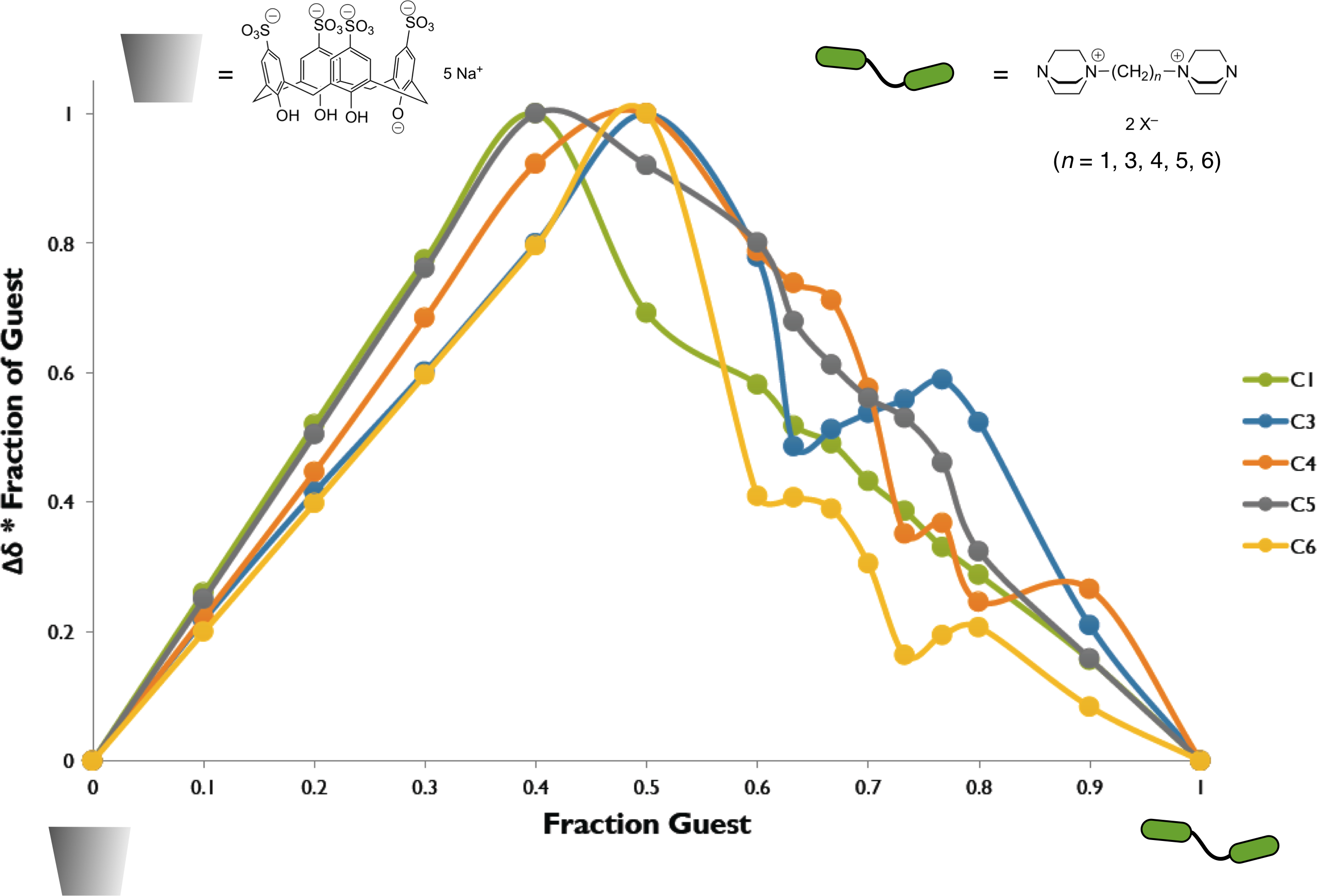
The stoichiometry of the complex or complexes formed between the host and diguest was probed by the method of continuous variation (Job plot). 1H NMR spectral signals from the diguest shift position upon interaction of the diguest and host molecules. The Job plots exhibit a maximum at or near a 1:1 ratio of H and G––G. At ratios higher than this (excess host) the plot appears as a continuous smooth curve. However, at lower ratios (excess diguest) the plot shows variations that may indicate multiple competing equilibria that include higher-order complexes. Such behavior has not been adequately modeled or explained, and further experiments are required.
Figure 1. Job plot for the interaction of sulfonated calixarene host with DABCO di-guest species. The 1H NMR chemical shift position for hydrogens on the di-guest varies with the ratio of host to di-guest.
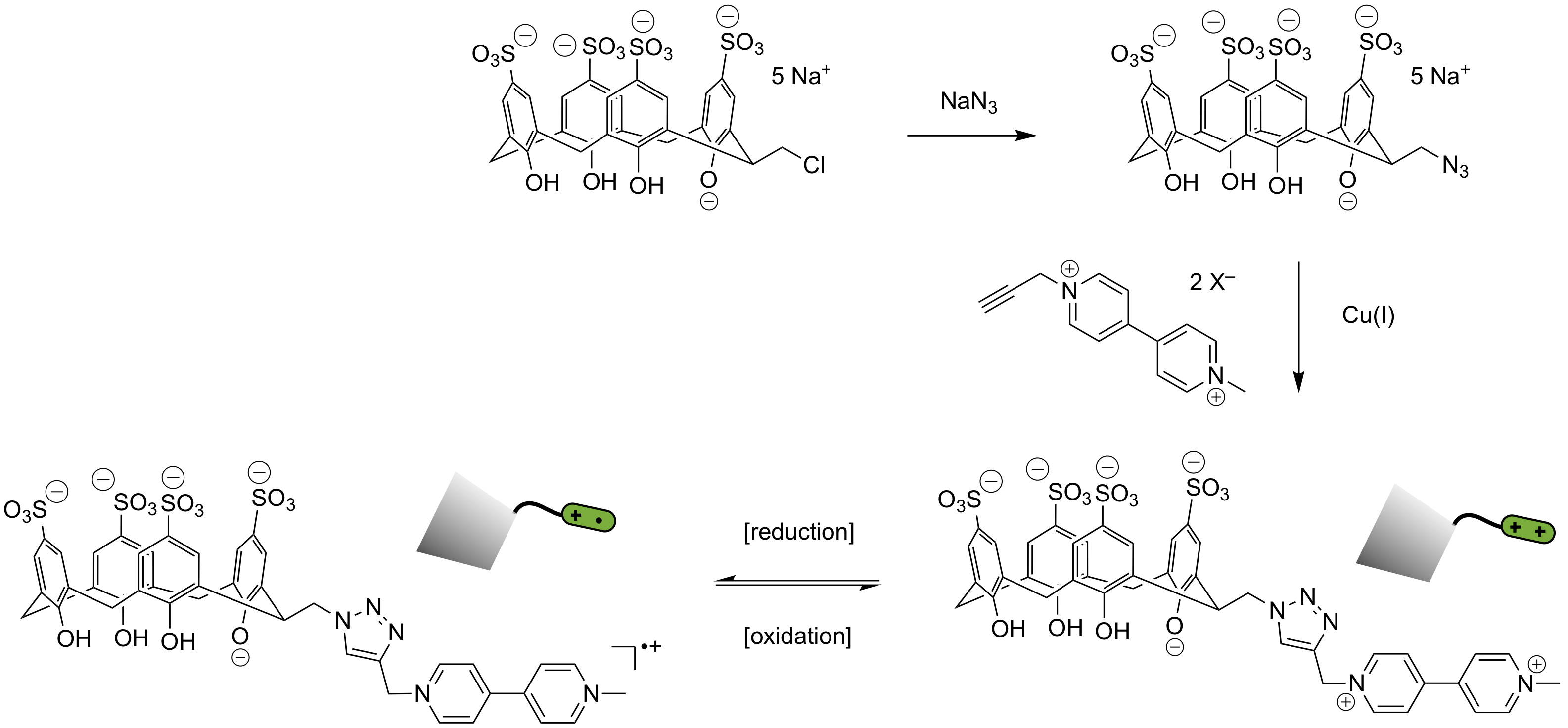
The second study involves the synthesis of a calixarene host with a guest attached at the end of a linker unit. The synthesis has been designed to be modular in nature as shown in the figure. First, a water-soluble sulfonated calixarene with a chloroalkyl tether will be transformed by substitution with sodium azide to give an azido tether. We have performed this successfully on a calixarene with a chlorobutyl tether. Further work will be with a shorter linker (chloromethyl) as shown in Figure 2, so as to end with an AB-monomer unlikely to do self-association.
By means of a copper(I)-catalyzed azide–alkyne cycloaddition the calixarene will be linked to a suitable guest moiety. In this case we are starting with a dialkylviologen unit as shown in Figure 2. This propargyl-containing species has recently been synthesized in our laboratory. The desired calixarene with a linked viologen unit will be suited to chemical or electrochemical reduction and in future work the supramolecular polymerization tendencies of the oxidized and reduced species will be investigated.
Figure 2. Route to AB-monomer via “click” reaction.