Reports: UNI254852-UNI2: Late Phanerozoic Evolution of Seawater Temperature and Sr/Ca: New Insights from Clumped Isotope Thermometry in Biogenic Apatite
Michael Lindgren Griffiths, PhD, William Paterson University
Project Summary
Gaining a better handle on past seawater Sr/Ca ratios is critical to improved understanding of various geologic and biogeochemical processes related to plate tectonics, weathering, diagenesis, and the carbon cycle. Despite significant advances in our understanding of long-term changes in the global ocean seawater Sr/Ca concentration via numerous proxy records, there are still significant discrepancies between the different organic and inorganic carbonate archives. These differences likely stem from the fact that the Sr/Ca of these carbonate proxies are influenced both by mineral formation temperature and the Sr/Ca of seawater from which the mineral forms. To circumvent these issues associated with seawater formation temperatures, we proposed to use “clumped isotope” thermometry in the biogenic phosphate of fossil shark teeth to effectively differentiate the influence of these two factors on the Sr/Ca.
Ancestral sharks are unique in that they have a globally robust and continuous fossil record since the late Cretaceous. Over the past decade, marine biogenic apatite—specifically enameloid (comprising the dense crown tissue) in modern and fossil shark teeth—has exhibited some success in providing a new tool for reconstructing the evolution of the world’s oceans. This is largely due to the fact that enameloid has been shown to accurately preserve the aqueous conditions of the seawater (i.e. isotope and elemental composition) at the time of tooth formation.
Results pertaining to the main objectives:
Objective 1: Establish the modern Δ47-temperature relationship in biogenic apatite from the teeth of both aquarium-reared and wild-caught sharks.
As noted in the proposal, clumped isotope thermometry is an emerging geochemical technique that is based on the thermodynamic preference of 13C and 18O to form bonds, or “clump”, with each other in the carbonate mineral lattice, and to date has primarily been used for analysis of mass 47 CO2 (13C18O16O). The basis for the clumping of these heavier isotopes into bonds with each other relies on the principles of quantum mechanical and statistical thermodynamics, which predict that multiply-substituted isotopologues of CO2 have lower free energies, and hence are more stable, than isotopologues with one or no heavy isotopes. This thermodynamic preference for clumped isotopologues in calcium carbonate can thus form the basis for a single-phase temperature proxy that is independent of bulk isotopic composition.
The first component of this work has been to establish a modern Δ47-temperature calibration curve using teeth from both aquarium-reared and wild-caught shark species from a range of ambient water temperatures; once completed, this calibration will be compared with other Δ47-temperature equations derived from both inorganic calcite and biogenic apatite. The initial part of the work has involved undergraduate students at William Paterson University drilling both the dentine and enameloid components of the modern shark teeth. Approximately 300-500 mg powdered bioapatite samples from a host of species were then acid washed to remove any organic material that can otherwise corrupt the geochemical signal. Once samples were cleaned, they were sent off to colleagues at UCLA (collaborator Dr. Robert Eagle) where the Δ47 measurements have been conducted. Preliminary results are highlighted in the table below.
Table 1. Preliminary clumped isotope results of wild-caught and aquarium-reared shark teeth. The encouraging results show that the Δ47-inferred body temperatures of the wild-caught teeth are within range of their known habitat temperature range. Most notably though, are the results of the aquarium-reared Sand Tiger Shark teeth (orange shaded row), with the Δ47-inferred body temperatures matching precisely the known temperature of the ambient temperature of the water tank. Based on these preliminary results, we are confident that the methods will be accurate when applied to the fossil specimens. In fact, initial results of the Megalodon species (green shaded row) are consistent with expected values based on the time period (late Miocene; 8-10 million years before present) and the projected size of the animal.
Species
| Locality
| #n analyses
| D 47
| Estimated body temperature (°C)
| D47 Temperature (°C)
|
Sand Tiger Shark
| Birmingham Aquarium (UK)
| 4
| 0.642±0.007
| 24
| 24.15±0.5
|
Tiger Shark
| Durban, South Africa
| 2
| 0.627±0.006
| 25-30
| 29.02±0.5
|
Mako
| Mid-Atlantic Continental Shelf
| 1
| 0.629±0.008
| 20-30
| 28.44
|
White Shark
| Durban, South Africa
| 2
| 0.605±0.007
| 20-40
| 34.8±13
|
Bull Shark
| Durban, South Africa
| 2
| 0.627±0.007
| 20-30
| 28.9±5.8
|
Megalodon
| North Carolina
| 1
| 0.612±0.006
|
| 32.5
|
Objective 2: Assess the potential effects of diagenesis on both the enameloid and dentine phases of the fossil shark tooth bioapatite, and assess the likelihood of alteration under different sedimentary conditions.
Numerous undergraduates at William Paterson University have been experimenting with the use of Scanning Electron Microscopy (SEM), Fourier Transform Infrared Spectroscopy (FTIR), and X-ray diffraction (XRD) to elucidate potential diagenetic alteration of the fossil bioapatite. Whilst this work is still in its infancy, these chemical and crystallographic analyses reveal differences between the fluoroapatite [Ca5(PO4)F] comprising the enameloid and dentine in both modern and fossil teeth. We attribute these differences to varying amounts of chemical substitutions of phosphate by carbonate and fluoride by hydroxide in the crystal lattice of the more porous and permeable dentine tooth tissues. This result is of little surprise though, and has been documented quite a bit in the literature. At face value, these results appear to suggest that the more robust/compact enameloid component of the modern and fossil tooth tissue is resistant to diagenetic alteration, and thus likely to have preserved the isotopic signature during formation.
Objective 3: Construct a record of bulk-ocean Sr/Casw using both clumped isotope thermometry and previously reported partition coefficients for Sr in biogenic apatite.
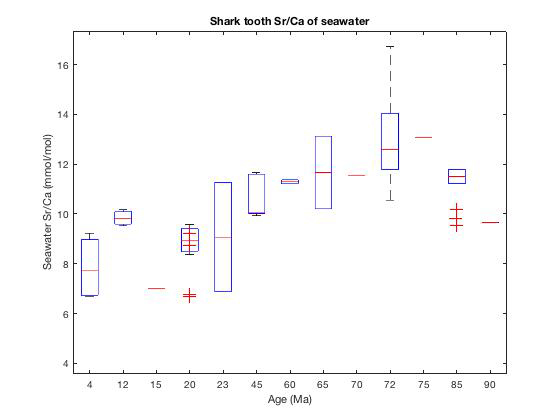
Results of this component of the work, conducted on an ICP-MS at Rutgers University, indicate that the Sr/Casw has overall declined since the late Cretaceous (~75 million years ago), a finding that is echoed in other marine fossil assemblages. Whilst this work is still in its infancy, we tentatively interpret the decline Sr/Casw to be a regionally (and potentially global) coherent signal, and as such, may provide a new record of Sr and Ca flux to the paleo-ocean (Fig. 1). However, these values may change depending upon the concomitant Δ47 temperature correction that may be applied once the clumped isotope analyses have been completed.
Fig. 1