Reports: ND555659-ND5: Understanding Microscopic- and Molecular-Level Mechanisms Involved in Wettability Alteration on Mineral Surfaces
Udo Becker, PhD, University of Michigan
1. Introduction
Understanding the molecular interaction between surface-active compounds in crude oil and mineral surfaces is needed to clarify the complex nature of wettability changes that take place during oil migration, production, and secondary or tertiary recovery. Wettability, or the enhanced affinity of the mineral surface for one fluid (often sea water) in the presence of, e.g., crude oil is a critical factor to consider at all stages of oil production because it can affect reserve estimates, yields, and determine the success of a recovery campaign.
Initially, we explored what properties of the surface cause one fluid to interact preferentially with the surface in the presence of another fluid, using Kelvin Probe Microscopy (applied for the first time on wettability) and advanced our understanding of the electronic properties of the surface as a major driver in wettability alteration. This led to a recent publication in the Journal of Physical Chemistry.
We are now focused on surface-active compounds in crude oil, which can change the wettability of reservoirs rocks from water-wet to oil-wet. Since neither the composition of any specific oil with respect to these compounds nor the exact mechanism behind making a surface more amenable towards adsorption of non-polar fractions in oil are known, prediction of wettability changes is often impossible. One group of such surface-active compounds are asphaltenes and we are testing which asphaltenes may change mineral surface wettability. The structure of asphaltenes is not fully deciphered but some key players may be phenolic groups that bind to the mineral surface and benzene rings that can interact with the non-polar oil.
Within this complexity, a common scenario stands out. Initially water-wet mineral surfaces are changed to oil-wet during oil migration. This change in wettability is carried out by surface-active compounds present in oil (i.e., asphaltenes), which disrupt the water film on the minerals and renders them oil-wet. Later during oil recovery, the return to more water-wet conditions on the reservoir becomes critical because the amount of oil that can be displaced from the porous space and that can flow out of the reservoir depends on it.
2. Approach
One method, used in the second year of funding, to determine where on the surface crude oil or asphaltenes therein attack specific surface sites is atomic force microscopy. AFM imaging of the adsorption of (1) crude oil, (2) asphaltenes previously separated from crude oil, and (3) hydroxyl functional groups in phenol onto calcite reveals attributes among these interactions that clarify sorption mechanisms and molecular structure of surface-reactive substances in crude oil.
The goal is to recognize correlations and distinctions among adsorption behaviors that explain molecular interactions. We chose a medium oil, an isolated asphaltene from a similar medium crude, and phenol. Previous studies have shown that functional groups consisting of N and O are concentrated on adsorbed asphaltenes and are important in the initial steps of surface-specific interactions. Phenol has a side group and a functional group found in asphaltene molecules. We started with a simple phenol that mimics the aromatic character of asphaltene with one hydroxyl group that binds to the calcite surface.
3. Selected Results
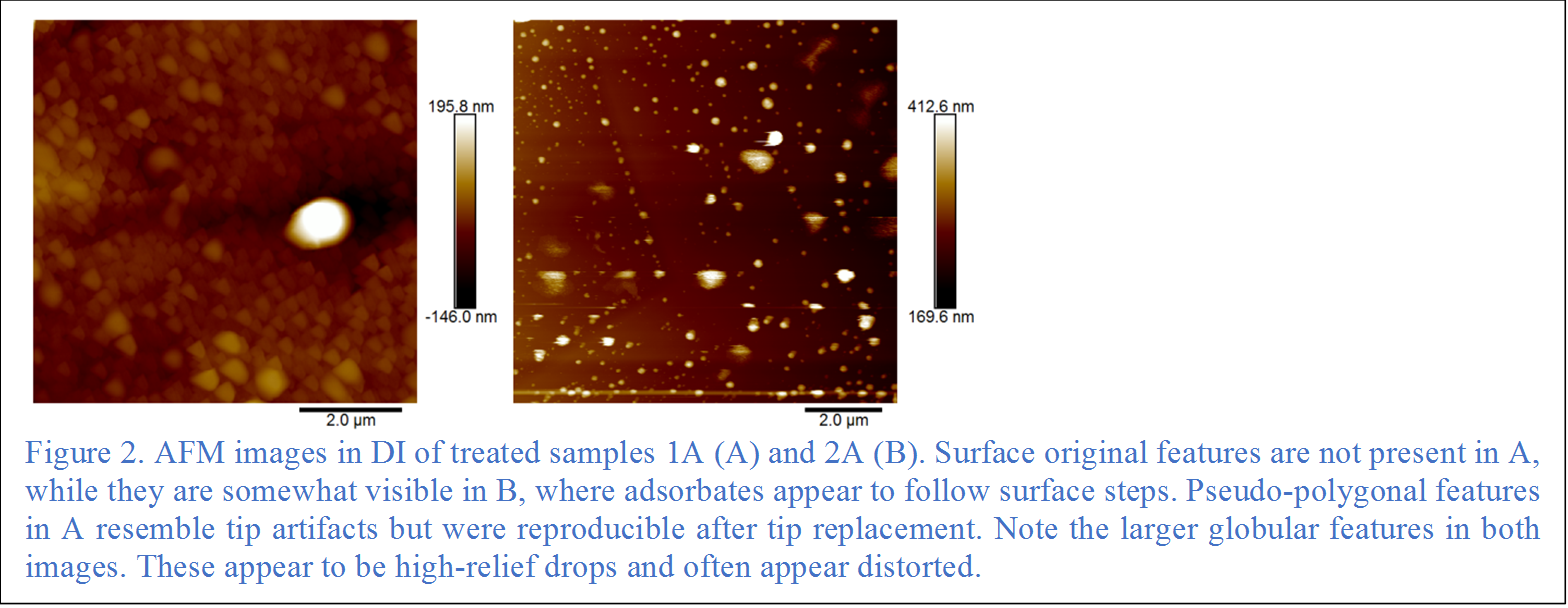
At the microscopic scale, medium crude oil adsorption is observed onto surface steps on the {10-14} face with nanoscopic droplets attaching every ~200 nm after 20 min reaction (Fig.1). Adsorption to terrace sites with no step preference and less organization is observed at longer reaction times (Fig. 2). Seawater conditioning enhances wettability changes, while brine and seawater have less impact under the conditions examined.
![Figure 1. (A) Height AFM image of a cleaved calcite after contact with crude oil. Red lines follow the traces of the [4 ̅41] and [481 ̅] steps joined at angles of 102° and 78° on the plane of the face. The red arrow points toward the larger and better organized adsorbates along the upper traces, tentatively identified as [4 ̅41]+ and [481 ̅]+, the obtuse step. (B) Schematics of a cross-section of the surface pit along the C-C’ direction showing the angular relation with the molecular layer underneath. Structurally non-equivalent steps are shown along [4 ̅41]− (part of the acute step [4 ̅41]− - [481 ̅]−) and [481 ̅]+ (part of the obtuse step [4 ̅41]+ - [481 ̅]+). The angular relation shown characterizes both growth (not present) and dissolution features on the {101 ̅4} face.](abimages/Paper_15192_abstract_38637_0.png) |
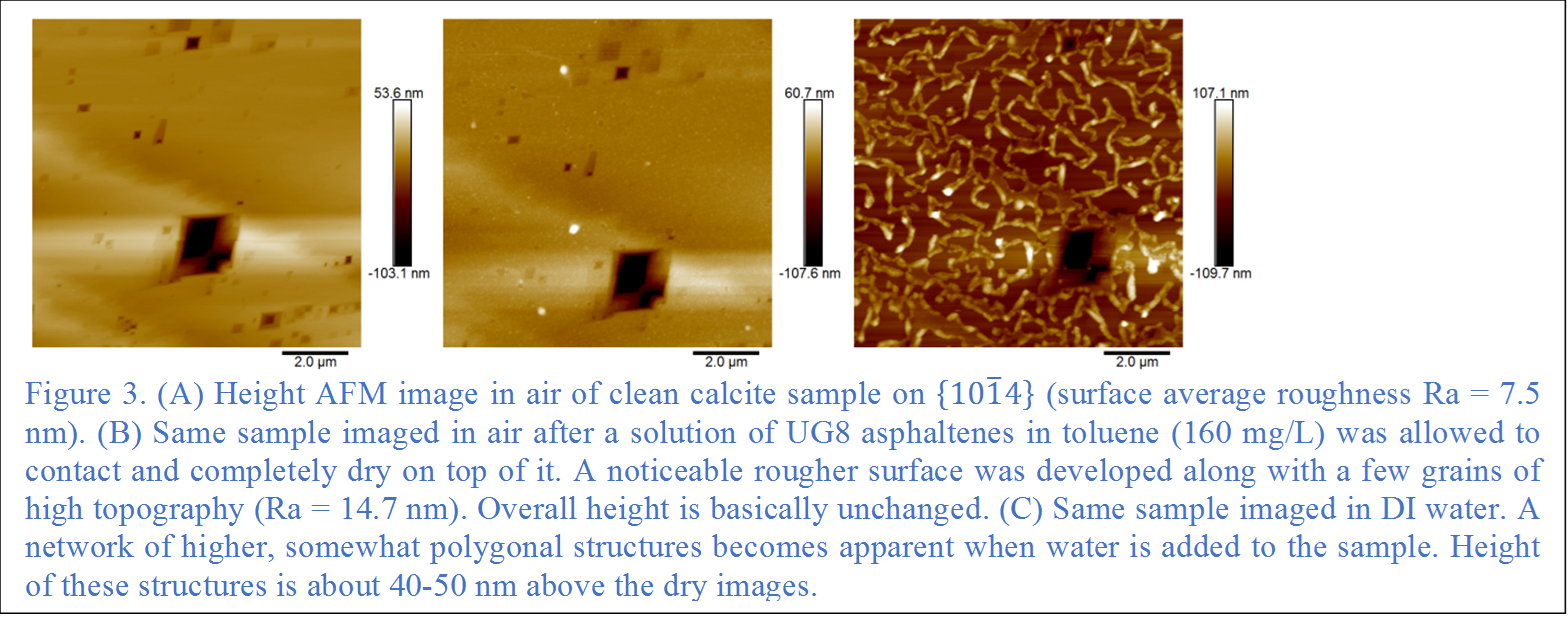
UG8, an asphaltene separated from a similar crude, shows semi-polygonal continuous structures that are in general alignment with the [010] step direction (Fig. 3). In contrast to reaction with crude oil, discrete colonization of parallel steps is not evident. Nano-aggregation of asphaltene molecules was required for directional adsorption to occur.
Hydroxyl groups on phenol in n-decane show adsorbates with no orientation or adsorption preference. Phenol in water show both individual droplet preferentially attached to steps in cleavage pits and semi-polygonal features that lack clear direction but are reminiscent of the adsorbates produced by asphaltenes.
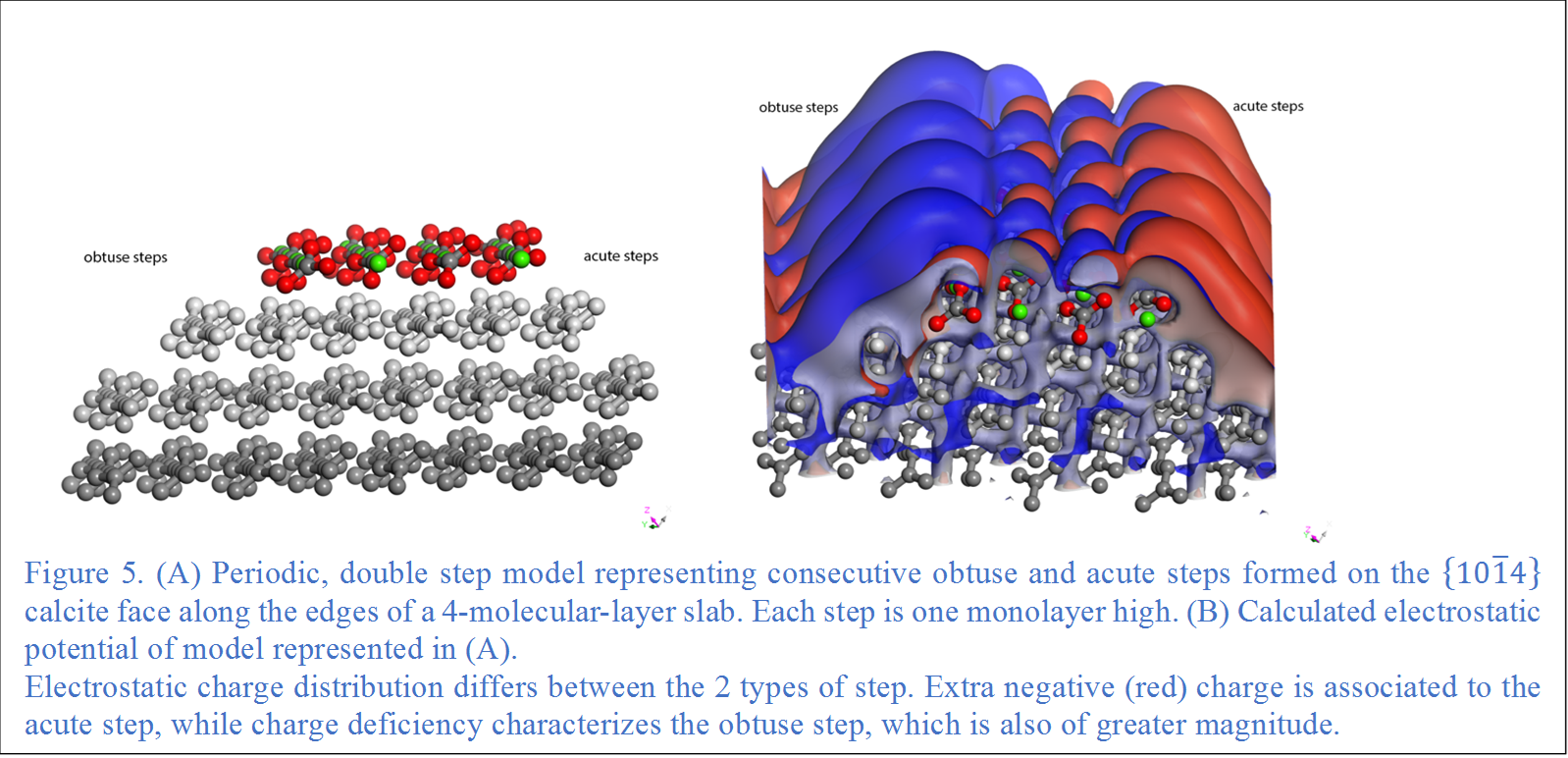
We considered the fundamental influence over adsorption that may be exerted by the charge distribution on the calcite surface by calculating electrostatic potential maps on a modelled etch pit (Fig. 4A). These simulations show larger areas of charge excess or deficiency that may influence the behavior of adsorbates beyond the individual atomic charges (Fig. 4B). Results show positive (blue) areas associated to both obtuse kinks and trace intersections, and negative (red) areas with excess charge associated to the acute steps. The largest surfaces are positive, appear associated to obtuse steps, and are likely more relevant at initiating surface interactions. The contrast and magnitude of the surface charge become more evident when the simulation of the steps is more complex (Fig. 5).
 |
4. Ongoing work and career impacts
![Figure 4. (A) Monolayer pit on the (colored) uppermost molecular layer of the calcite {101 ̅4} face with steps along directions [4 ̅41] and [481 ̅]. View approximately normal to {101 ̅4} face. (B) Electrostatic potential of configuration shown in (A).
Upper molecular layer – C grey, O red, Ca green (except one yellow corner atom to relate the images). Middle molecular layer – all atoms light grey; lower molecular layer – all atoms dark grey. Continuous lines are acute steps, dashed lines are obtuse steps. The intersection of the step traces creates obtuse (upper-right and lower-left) and acute (lower-right and upper-left) kinks on the surface that differ in atomic configuration. Obtuse kinks present Ca atoms to the open pit, while acute kinks present CO3 atoms (highlighted in A). Obtuse pit kinks result in positive electrostatic potential surfaces (blue in B). Acute pit kinks produced negative electrostatic potential surfaces (red in B). The upper-right kink in the pit is the intersection of [4 ̅41] and [481 ̅] steps that grow or dissolve at an acute angle with respect to the lower molecular layer (continuous lines). The lower-left kink is the intersection of steps that grow or dissolve at an obtuse angle with respect to the lower molecular layer (dashed lines).](abimages/Paper_15192_abstract_38640_0.png) |
Kelvin-Probe-Force Microscopy experiments with HMDS and reservoir-relevant minerals (e.g., quartz and clay minerals) as well as adsorption experiments with other proxies of surface-active compounds in crude oil (e.g., 1-naphtol) have been and will be carried out. Additionally, calcite surface changes associated to asphaltene exposure at high pressure and temperature (at ranges usually employed in core experiments) is ongoing and will be useful to compare with experiments at normal conditions.
The opportunity provided by the PRF research funds has resulted in the development and expansion of knowledge, methods, and techniques relevant to petroleum engineering and hydrocarbon reservoir characterization. We have used a diverse research approach focusing in turn on each one of the factors relevant to wettability changes using leading-edge experimental and computational approaches. As a result, our group has expanded its expertise and increased its research potential. The research has led to a publication and two chapters in the thesis of a recent Ph.D. graduate; it was also crucial in this student landing a job. A current student has taken advantage of the wettability project to learn AFM techniques and computational approaches. At least two more publications are expected to be completed in 2018.