Reports: ND355495-ND3: Homogeneous Nanoparticle Catalysis: Synthesis, Structural Characterization, and Catalysis Applications of Molecular Transition Metal Clusters
Jonathan Owen, PhD, Columbia University
Introduction. We seek control the structure of graphite derived electrocatalysts with atomic precision. Such structures can provide unprecedented levels of selectivity in otherwise challenging transformations like CO2 reduction to hydrocarbon fuels. Through catalytic gasification of graphite (Figure 1) nanometer wide trenches can be etched into graphite that are atomically straight over μm lengths. Fine structure control over these length scales is a unique feature of these trenches. We aim to functionalize the trench edges with catalysts for a variety of electrochemical transformations. Highly dense active sites that are directly contacted by the electrode would be a broadly impactful electrochemical platform. We are studying the processes that control edge initiation and propagation to gain improved control over the trench orientation, width, and mean length. With these edges in hand, we are testing their reactivity with azide reagents to append amine functional groups in an ordered fashion. These edges are being characterized with a variety of techniques, including scanning electron microscopy (SEM), atomic force microscopy (AFM), Raman microscopy, and scanning tunneling microscopy (STM).
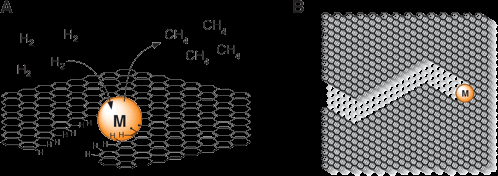
Figure 1. A) A 0.5 nm transition metal catalyst cluster etching a channel in single layer graphene. Hydrogenolysis of the graphene lattice leads to the excision of carbon atoms via the loss of methane. B) A cartoon illustrating ‘edge controlled’ etching of graphene leading, in this example, to exclusive production of zig zag edge structure.
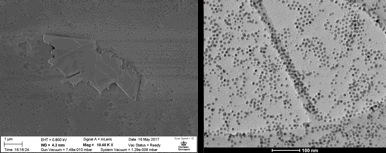
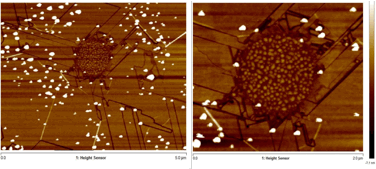
Controlling etching through particle synthesis and patterning. The width of the trenches cut via gasification is determined by the size of the transition metal particle gasification catalyst. Through colloidal synthesis the size of the metal particles can be tightly controlled. Cobalt metal nanoparticles were synthesized by established literature methods1 and used as etching catalysts. The size control is limited somewhat by the agglomeration of particles under the gasification conditions. Nonetheless, preformed cobalt nanocrystals produce samples with long straight channels as can be seen by SEM (Figure 2). Trench initiation in this sample, and in others, occurs at edges. We are using this principle to control the point of initiation by patterning holes in graphene. Trenches initiated at well defined patterns, in principle, would allow controlled production of arrays, superlattices, and other structures of etched graphene shapes. Patterning defects through electron beam lithography has proven to be an effective method of inducing catalyst initiation, as seen in the AFM micrograph seen in Figure 2, where trenches extend from a 1µm hole patterned by electron-beam lithography.
Figure 2. (Left) SEM Micrograph of graphite flake etched with cobalt nanoparticles. (Right) TEM micrograph of colloidally synthesized nanoparticles.
Figure 3. AFM MicrogrAph of a lithographically patterned hole with metal particle etched trenches emanating outward.
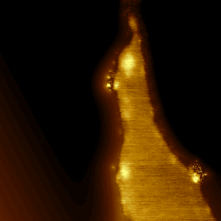
In-situ functionalization via reactive gases. To obtain nitrogen functionalized edges we performed gasification reactions with NH3 rather than dihydrogen. We hypothesized this would provide amine terminated edges rather than what is presumed to be a hydrogen terminated edge. Experiments with ammonia resulted in two forms of gasification, (1) unselective etching of pits (likely the result of direct reaction of NH3 with the carbon) and catalytic trench formation from gasification, possibly via H2 produced by NH3 cracking. XPS analysis showed N signal incorporated into the graphite, however a large oxygen signal was also present, casting doubt on the direct nitrogenation of the graphite (NH3 replacement of graphite oxide is known2). Collaborators in the Pasupathy lab at Columbia University performed STM analysis of the substrate with nitrogen XPS signal and saw regions of increased electron density shown in Figure 4, indicating nitrogen incorporation. These bright spots, however, were not seen on the straight edges of the etched trenches, and may have incorporated at other defects containing oxygen.
Post etching functionalization via azido reactions. Due to the limitations of direct functionalization with reactive gases, including low selectivity, dangerous precursors, and harsh reaction conditions, a new strategy of post etching functionalization is proposed. We aim to study graphite functionalization using azide and diazonium reagents, to produce functionality on the long, straight etched edges.3 The selectivity will be studied using STM and will allow us to address selectivity issues in the reactivity of graphite with unprecedented detail.
Figure 4. 50 nm wide STM micrograph of NH3 etched HOPG recorded at 150pA, 2V. Bright spots are nitrogen atoms.
Attachment of molecular catalyst to graphene nanoribbon electrode. After protocols are developed for the precise control and characterization of graphitic edges, we aim to elaborate the nitrogen functionalized edge with catalysts molecules. Tuning the metal identity and ligand shell will allow for selectivity of carbon based products and the efficiency of the catalytic processes. We look to explore how changing the linkage between the molecular catalyst and the electrode can affect the efficiency, selectivity, and products of CO2 reduction with a high degree of structural clarity.
References
(1) Puntes, V. F.; Krishnan, K. M.; Alivisatos, A. P. Science 2001, 291 (5511), 2115–2117.
(2) Li, X.; Wang, H.; Robinson, J. T.; Sanchez, H.; Diankov, G.; Dai, H. Journal of the American Chemical Society 2009, 131 (43), 15939–15944.
(3) Liu, L.-H.; Yan, M. Acc. Chem. Res. 2010, 43 (11), 1434–1443.