Reports: UNI356090-UNI3: Towards Transition Metal Catalyzed Oxidative Functionalization of Alkanes by Molecular Oxygen
Jason M. Keith, PhD, Colgate University
Purpose:
The overall goal of this work is to develop catalysts that use oxygen (O2) to oxidize alkanes such as methane gas (CH4) to liquid methanol (CH3OH), while avoiding species such as formaldehyde (CH2O), formic acid (CHOOH) and carbon dioxide (CO2). This would drastically change access to the major component of natural gas (~95%) by converting a gaseous fuel with high barriers for transportation to a liquid that could be easily transported. While the C-H activation step involved has not been examined, catalytic activation of O2, a fundamental step in this and other important organic transformations has been examined in detail computationally with the help of undergraduate researchers and several publications are upcoming.
Student Involvement:
A total of 9 students have participated in this work to date
Clare Bannon:
Fall 2016 to Spring 2017
Graduated Spring 2017
Employed at Cabot Corp.
Samuel Kim:
Fall 2016 to Spring 2017
Graduated Spring 2017
Taking a gap year before medical school
Garrett Esper:
Summer 2016, Fall 2017 to current
Expected graduation Spring 2018
Accepted to Medical School
Sydney Loria:
Summer 2016, Spring 2018
Expected graduation Spring 2018
Accepted to Medical School
Markus Miranda:
Summer 2016 to current
Expected graduation Spring 2019
Plans to attend graduate school in chemistry
Harrison Chen:
Summer 2017 to current
Expected graduation Spring 2019
Plans undetermined
Oliver Harris:
Summer 2017 to current
Expected graduation Spring 2019
Plans undetermined
Matthew Bousquet:
Summer 2017 to current
Expected graduation Spring 2020
Plans undetermined
Recent results:
As outlined previously the Keith group is examining insertion of molecular oxygen into M-R bonds (R = H, CH3) in an effort to understand mechanistic pathways for selective alkane oxidation by transition metal catalysts. The group examines these mechanistic pathways computationally with Density Functional Theory. Towards this goal the group has focused on specific reactions:
1. Investigation the Competition between the Hydrogen Atom Abstraction (HAA) and Radical Chain (RC) mechanisms for O2 insertion in Rh-H bonds
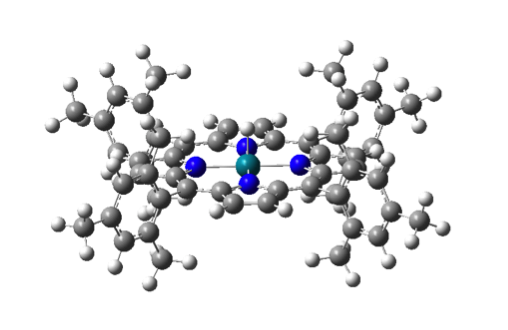
Figure 1. (left) (porphyrin)RhIII-H ;(right) [cyclam-RhIII(H2O)-H]2+.
This project has been ongoing since summer of 2016. Preliminary results suggest that the porphyrin complex undergoes O2 insertion through the HAA mechanism while the cyclam complex undergoes this reaction through a RC process. The distinction appears to be due to the steric bulk of the four mesityl groups on the porphryin complex preventing radical propagation steps involving two Rh centers. Continued work is focused on examining the photochemistry involved in these reactions. Undergraduate researchers have been involved in 100% of this work and are currently working to finished this project with publication submission expected in late 2018.
Cui, W.; Wayland, B.B. J. Am. Chem. Soc. 2006, 128, 10350-10351.
Bakac, A. J. Am. Chem. Soc. 1997, 119, 10726-10731.
Bakac, A. J. Photoch Photobio A 2000, 132, 87-89.
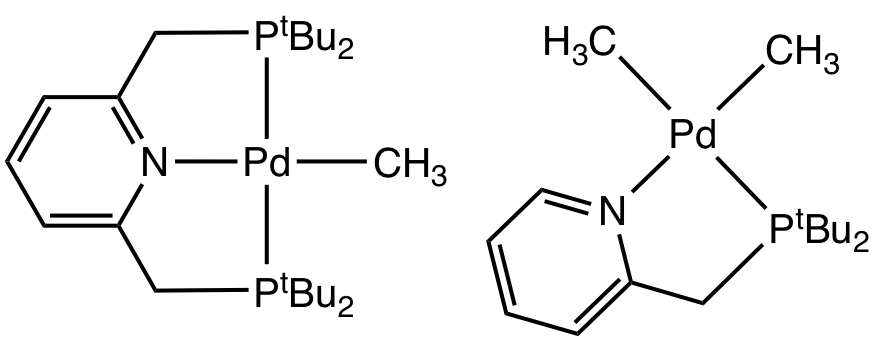
2. Mechanistic Investigation of Radical Cata-lyzed Insertion of Molecular Oxygen into a Pd-Me bond: Pd(III) versus Pd(I)
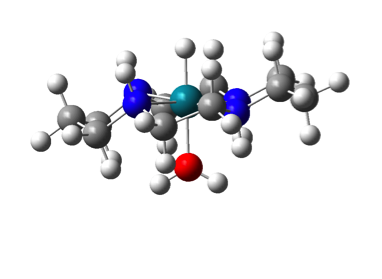
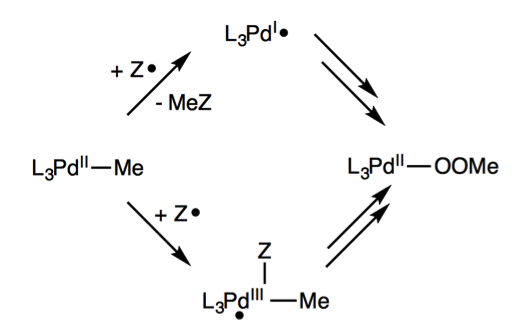
Figure 2. (left) Pd(II) methyl complex (right) Scheme for radical (i.e. 1 electron) pathways for insertion of O2 into Pd-Me bond.
This project was completed in 2017 and is currently being written up for publication. This work examined the previously rare Pd(I) and Pd(III) complexes that would be observed through a radical chain process. Through this work the SH2 mechanism was examined and found to exist for this process although with high barriers.
Alternatively this reaction was found to proceed through radical attack on the Pd center to form a 5-corrdinate Pd(III) radical species which would then undergo rearrangement resulting in the loss of methyl radicals. This turnstile-like mechanism is consistent with experimental finding. One interesting discovery from this work was the reaction of molecular oxygen (a diradical) with a radical species to activate the oxygen radical. This in effect makes molecular oxygen more radical-like and promotes the overall reaction. The group is already seeing applications of this idea to other studies.
Boisvert, L.; Denney, M. C.; Hanson, S. K.; Goldberg, K. I. J. Am. Chem. Soc. 2009, 131, 15802-158814.
3. Investigation the Competition between the Hydrogen Atom Abstraction (HAA) and Reductive Elimination/Oxidative Addition (RE) mechanisms for O2 insertion in Rh-H bonds
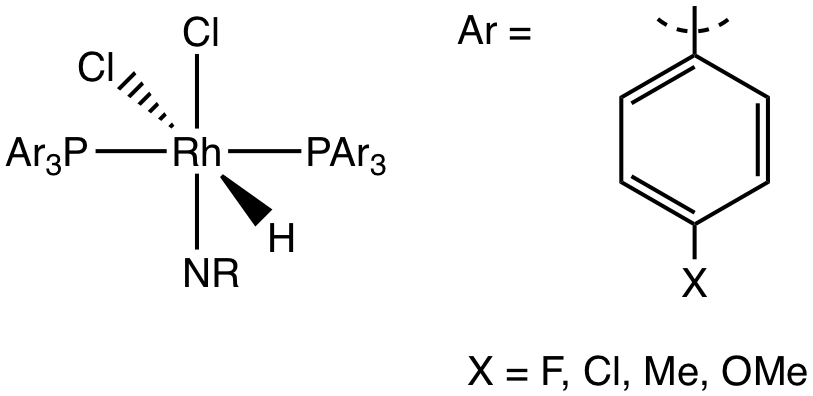
Figure 3. Octahedral Rh(III) hydride complex.
Research was begun on this project in Summer 2017 and is ongoing. Students have evaluated a competition between HAA and RE for an octahedral Rh(III) hydride complex which demonstrates kinetics consistent with HAA. Key aspects of this system allow for the examination of effects of stereochemistry on the reaction, as the complex can exist in two stereoisomers (cis and trans dicholoride), as well as the effects of modifying the electronic properties on the ancillary ligands.
Halbach, R. L.; Teets, T. T.; Nocera, D. G. Inorg. Chem. 2015, 54, 7335-7344.
4. Investigation into photoinduced radical formation in Pd and Pt Methyl Complexes
Figure 4. Square planar Pd(II) complexes reported to produce photoinduced radical reactions.
Work has been begun investigating two systems that demonstrate photoinduced insertion of molecular oxygen into Pd-Me bonds. Prelimary investigations have focused on TDDFT simulations of the UV-Vis spectra. Work is ongoing.
Smoll, K. A.; Kaminsky, W.; Goldberg, K. I.; Organometallics 2017, 36, 1213-1216.
Grice, K. A.; Goldberg, K. I.; Organometallics 2009, 28, 953-955.