Reports: ND155220-ND1: Strained Cyclic Allenes: Novel Methods for Generation, New Trapping Reactions, and Domino/Cascade Processes
Frederick G. West, University of Alberta
This project seeks to develop new, mild methods for the generation of high-energy strained cyclic allenes, and to discover efficient, new reactivity driven by strain relief. In particular, we are interested in intercepting the reactive allenes via pericyclic processes, allowing for the establishment of multiple new strategic bonds and stereogenic centers. The combination of efficient generation methods and novel trapping modalities will then allow the application of this chemistry in the synthesis of complex target molecules.
This year, we have focused on two main threads: (1) alternative methods for generating the high-energy cyclic allene under the mildest possible conditions; and (2) generalization of intramolecular trapping processes.
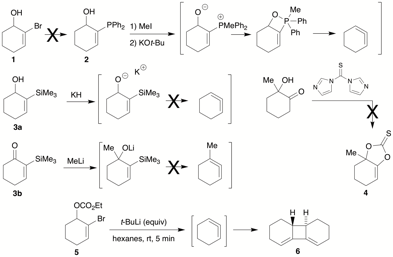
The first aspect explored several techniques for generation of an olefin wherein a highly favorable by product was generated (Scheme 1). These included Wittig reaction, Peterson olefination, Corey-Winter reaction, and lithium-halogen exchange/elimination. The Wittig approach required access to 2-phosphinyl cyclohexenol 2, which was to be formed from 2-bromo cyclohexenol 1. However, lithium-halogen exchange followed by phophinylation was unsuccessful. On the other hand, we were able to obtain the corresponding 2-silyl cyclohexenol 3a or 2-silyl cyclohexanone 3b by a similar route. Subsequent treatment with potassium base (3a) or 1,2-addition of methyl (3b) set the stage for the Peterson step; however, upon treatment with base this compound failed to generate the olefin, instead undergoing uncharacterized silyl-transfer processes. Conversion of the alcohol to a leaving group and treatment with fluoride also failed to effect elimination. Corey-Winter substrate 4 could not be formed from the corresponding hydroxyketone, so we were unable to test this approach. However, we discovered that various allylic leaving groups adjacent to a cyclohexenyl bromide underwent elimination to form the allene, which then dimerized as in 5→6.
Scheme 1
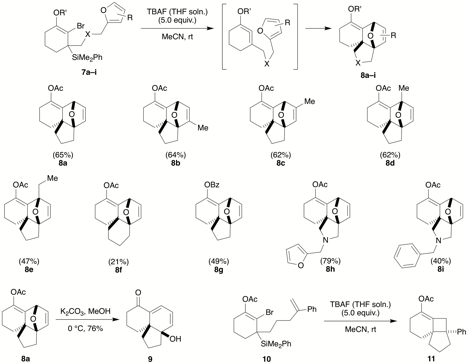
In the previous reporting period, we found that tethered furans efficiently trapped the transient cyclic allene. We have now generalized this to a variety of substrates (7→8), varying the tether and the furan substitution pattern (Scheme 2). We also showed that the strained dihydrofuran bridging ether could undergo clean eliminative opening upon treatment with mild basic conditions (8a→9). Moreover, this sort of efficient intramolecular trapping is not limited to Diels-Alder processes; a simple styrene trap also reacts cleanly with the cyclic allene to generate an angularly fused tricyclic 6-4-5 ring system suitable for possible application to sesquiterpene natural product targets (e.g., 10→11).
Scheme 2
In the future, we hope to generalize the intramolecular chemistry and apply it to natural product synthesis, and also explore heterocyclic variations on 1,2-cyclohexadiene.