Reports: DNI955467-DNI9: Nonaqueous Electrochemical Activation of Methane under Ambient Conditions for High-Value Liquid Fuel Production
Bingjun Xu, PhD, University of Delaware
The overarching goal of this project is to efficiently activate methane to higher value liquid fuel and chemicals. We propose to selectively activate C-H bonds in methane via a free radical cation-mediated activation pathway in nonaqueous media. Technical difficulties were encountered in the first year of the funding period, and as mentioned in the last technical report, we decided to pursue an alternative pathway to activate methane to valuable liquid products, i.e., dehydroaromatization (DHA) coupled with reactive hydrogen separation via chemical looping.
DHA converts methane into aromatics, e.g., benzene, and hydrogen with a theoretical 100% atom efficiency toward valuable aromatic compounds:
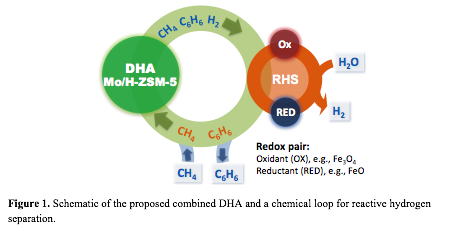
High selectivity for benzene (>80%) was reported on Mo supported HZSM-5 bifunctional catalysts. However, DHA of methane is severely limited by the thermodynamics, with an equilibrium conversion of methane at 700 °C below 15%. We propose to reactively separate hydrogen produced in the DHA step by coupling with a chemical loop involving a metal oxide redox pair, i.e., Fe3O4/FeO. Hydrogen is removed from the DHA effluent by reacting with Fe3O4 to produce FeO, while it is recovered in a separate step by exposing FeO to water (Figure 1).
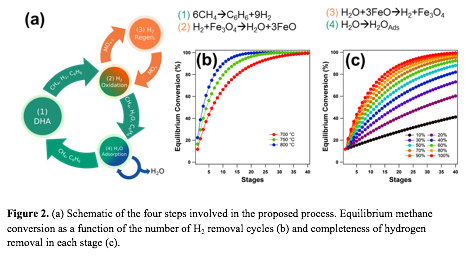
During the second year of the funded period, we successfully verified the four-step process to enhance the aromatics yield from DHA (Figure 2a): 1) DHA of methane, 2) hydrogen removal by reacting with Fe3O4, 3) hydrogen recovery by reacting FeO with water, and 4) water removal. Thermodynamic calculations show that the equilibrium conversion of methane increases steadily with the number of H2 removal cycles, or stages (Figure 2b). Further, more complete the hydrogen removal leads to more complete restoration of the thermodynamic driving force for DHA of methane, i.e., less stages needed to achieve a given methane conversion (Figure 2c). We also confirmed the feasibility of each of the four proposed steps:
 Step
1. DHA of methane on Mo/HZSM-5: The DHA step in the
first cycle is identical to the conventional DHA of methane, with methane being
the only hydrocarbon in the feed. We synthesized Mo/HZSM-5 (2wt % of Mo, Si/Al
= 15) by incipient wetness impregnation followed by calcination. The catalyst
was carburized in a 1:1 mixture of hydrogen and methane prior to reaction, and
evaluated in a CH4/N2 (95%/5%) stream at 700 °C.
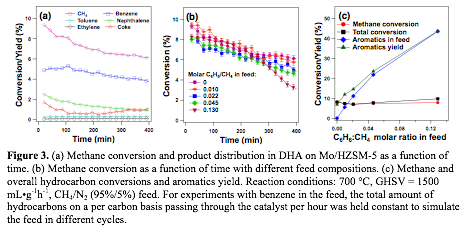
The methane conversion and product distribution within 400 min in our
preliminary experiments are consistent with previous reports on the same
catalyst under similar conditions (Figure 3a). We simulated the
feed after 1st stage by using a mixture of methane and aromatic compounds,
which are similar to the effluent stream from methane DHA. Experiments with the
hydrocarbon mixture with the molar ratio of benzene/methane corresponding to
feed compositions for 2nd to 6th stages show similar
methane conversions (Figure 3b), indicating that the thermodynamic driving
force of DHA is indeed restored after the hydrogen removal. It can be estimated
that the upper bound of total aromatics yield through repeated cycling with the
current catalyst is ~45% (Figure 3c), which is 4-5 times higher than that the
state-of-the-art yield of aromatics in one-pass reactors.
Step
1. DHA of methane on Mo/HZSM-5: The DHA step in the
first cycle is identical to the conventional DHA of methane, with methane being
the only hydrocarbon in the feed. We synthesized Mo/HZSM-5 (2wt % of Mo, Si/Al
= 15) by incipient wetness impregnation followed by calcination. The catalyst
was carburized in a 1:1 mixture of hydrogen and methane prior to reaction, and
evaluated in a CH4/N2 (95%/5%) stream at 700 °C.
The methane conversion and product distribution within 400 min in our
preliminary experiments are consistent with previous reports on the same
catalyst under similar conditions (Figure 3a). We simulated the
feed after 1st stage by using a mixture of methane and aromatic compounds,
which are similar to the effluent stream from methane DHA. Experiments with the
hydrocarbon mixture with the molar ratio of benzene/methane corresponding to
feed compositions for 2nd to 6th stages show similar
methane conversions (Figure 3b), indicating that the thermodynamic driving
force of DHA is indeed restored after the hydrogen removal. It can be estimated
that the upper bound of total aromatics yield through repeated cycling with the
current catalyst is ~45% (Figure 3c), which is 4-5 times higher than that the
state-of-the-art yield of aromatics in one-pass reactors.
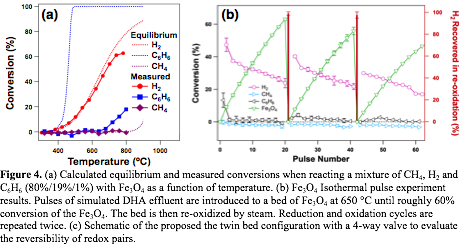
Steps 2&3. Hydrogen removal and regeneration via a chemical loop with FeO/Fe3O4: Pulse experiments on Fe3O4, in which 0.3 mL pluses of a CH4, H2 and C6H6 mixture were injected to a bed of Fe3O4 at different temperatures, show that hydrogen is selectively consumed in 400-650 °C range, in which no detectable level of methane or benzene is reacted (Figure 4a). The selective hydrogen oxidation has been confirmed at the benzene volume percentage in the feed ranging from 1% to 8%. While the measured conversions of hydrogen and methane are generally consistent with the thermodynamic predictions (solid and dotted lines in Figure 4a, respectively). The measured onset temperature for benzene conversion (670 °C) is almost 300 °C higher than predicted by thermodynamic calculations, suggesting a significant activation barrier for its activation on Fe3O4. Hydrogen can be regenerated by exposing FeO, produced via reducing Fe3O4 in the hydrogen oxidation step, to water at 700 °C, demonstrating the reversibility of the FeO/Fe3O4 redox pair (Figure 4b).
Step 4. Water removal from the effluent from the hydrogen oxidation step: The majority of the water produced from the hydrogen oxidation step needs be removed to ensure the high selectivity for aromatics in DHA and subsequent hydrogen oxidation steps. We show that zeolites 4A and 5A, to remove water from the effluent from the hydrogen oxidation step (Step 2).
Combining all four steps, the proposed cycle has the potential to drastically enhance the aromatics yield from methane DHA (by up to a factor of 4 based on thermodynamic equilibrium).
The support from ACS PRF has been a key enabling factor in jump-starting my newly established research program at University of Delaware. In this project, we have explored two distinctive approaches in methane activation, which is a major challenge in the petroleum research field. Although the effort to electrochemically upgrade methane was unsuccessful, we have acquired expertise in several key electrochemical and analytic techniques, e.g., cyclic voltammetry and mass spectrometry. The combination of catalytic hydrocarbon chemistry and chemical looping technology has developed into a key thrust in my research group.











