Reports: DNI1055708-DNI10: Syntheses of Polycarbonyl Aromatic Compounds for New Redox Materials
Xiulei Ji, PhD, Oregon State University
Using the PRF fund resources again this year, we have demonstrated, for the first time, a trend in polycyclic aromatic hydrocarbons (PAHs) - crystalline and readily available coronene, perylene, and triphenylene - exhibiting highly reversible anion-storage properties. The exploration of electrode materials in the paradigm of battery research has been shifting towards finding materials that are completely renewable, do not contain any transition metals, and are readily available.
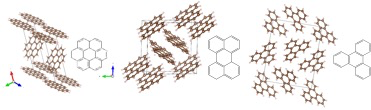
Figure 1 shows the crystal structures of the PAHs simulated using VESTA. As one can see, as we decrease the conjugation size and the size of the molecule, the number of edge sites increases per unit cell. This is important because we performed first principles computational studies that show the preferred sites of anions to be on the edge sites of the PAHs as opposed to being in between two molecules (Figure 2). This trend was hypothesized considering the different conjugation size of the molecules, the different densities, and the different number of edge sites, the more edge sites, the more sites the anions could store in, meaning the higher capacities could be. Figure 3 is showing the trend in the PAHs in terms of their performance in storage capacity, which coincide with the theoretical capacity increase as the molecule size decreases, and confirms our hypothesis.
Figure 3 shows the charge/discharge profiles for the PAHs for different cycles, where the plateau behavior is well maintained in coronene and perylene however not for triphenylene. This is due to the high solubility of the material in the electrolyte. Cyclic voltammetry (CV) (Figure 3b) results are also shows which high reversibility in the first 10 cycles for coronene and perylene, however, not for triphenylene once again.
Figure 1. Crystal structures of the PAHs studied simulated using VESTA. From left to right – coronene, perylene, and triphenylene. (manuscript under preparation)
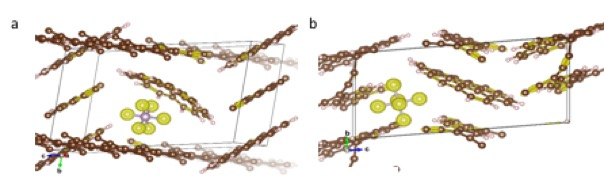 |
Figure 2. Simulations of the PF6- anions inserted into the coronene structure. a. PF6- anions inserted in between coronene molecules. b. PF6- anions inserted along the edges of the coronene molecules. (manuscript under preparation)
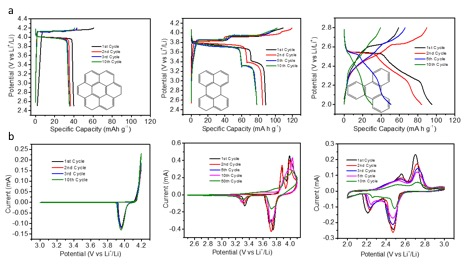 |
Figure 3. a. Galvanostatic charge/discharge profiles of the coronene, perylene and triphenylene (manuscript under preparation)
Another project that was supported by the PRF Fund was a project using a carbonyl group functionalized PAH. Here, we, for the first time, report that 3,4,9,10-perylenetetracaboxilicdianhydride (PTCDA), an inexpensive organic red pigment, contracts when hosting Mg-ions, Ca-ions, and hydronium-ions.
Previous data we presented showed the investigation of rate capability and cycling performance in three-electrode cells. PTCDA exhibited good rate capability despite its nature of being a semiconductor. At a high rate of 500 mA g-1 or 3.7 C, a capacity of 75 mA h g-1 is still retained. This may have to do with its spacious inter-plane space and a lack of ionic bonds in the structure, where ion-migration is not impeded. Relatively stable cycling was observed with capacity retention of 80% after 100 cycles at 20 mA g-1 and the coulombic efficiency goes up and stays above 90% after 15 cycles.
The electrochemical and structural characterization of the PTCDA electrode continued and gave us new insights into its potential for storage of different ions. We further show the electrochemical performance of PTCDA storing Mg-ions vs its storage of Na- and K-ions.
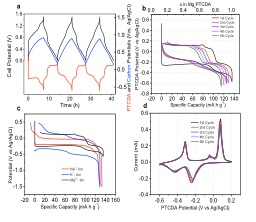 |
Figure 4. (a) The first three cycles of three-electrode cell measurements: full-cell voltage profiles (black) along with profiles of PTCDA electrode (red) and activated carbon electrode (blue), respectively, at a current rate of 20 mA g-1. (b) Galvanostatic magnesiation/demagnesiation potential profiles of the first five cycles of the PTCDA electrode at a current density of 20 mA g-1 in a potential range of -0.6 – 0.2 V vs Ag/AgCl reference electrode. c, Galvanostatic potential profiles of the first cycles of the PTCDA electrode for storing Na-ions, K-ions, and Mg-ions at current rates of 10 mA g-1, 20 mA g-1, and 20 mA g-1, respectively. (d) Cyclic voltammetry profiles of the first five cycles of the Mg-PTCDA electrode at a scan rate of 0.1 mV s-
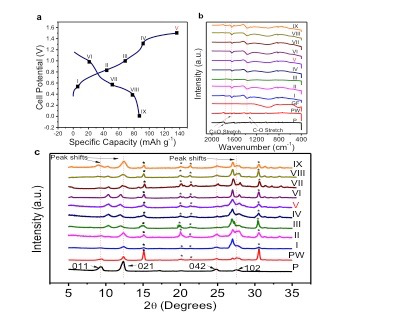
To investigate the evolving structure of the PTCDA crystal upon Mg-ion storage, ex situ x-ray diffraction (XRD) patterns were collected at different SOC in the first cycle (Figure 5c).
The contraction phenomenon is also well supported by simulation studies as the calculated distance between the two oxygen atoms that chelate one Mg2+ decreases from 4.05 to 3.41 ° from once the Mg-ion is present, thus shortening the d-spacing between (011) planes. We hypothesize that the disparity between the situations of (011) and (021) planes, where the (021) planes expanded due to the fact that two Mg2+ ions are in between the (021) planes, as opposed to just one between the (011) planes per unit cell. Interestingly, the (102) peak splits into higher and lower 2θ positions during magnesiation, meaning that some (102) planes expand and the other contract. This can be rationalized by examining the spatial distribution of the inserted Mg-ions and arrangement of the (102) planes, where two Mg2+ ions are inserted between every other (102) layers per unit cell, hence the planes with Mg2+ ions in between expand, and the planes without contracted as they are pushed by the expanded planes. Our results may open up a new avenue for charge storage with wide applications.
Figure 5.a) First-cycle charge/discharge potential profiles, where different SOC is marked. The Roman Numeral V is in red representing a fully charged electrode. b)-c) Ex situ FT-IR spectra and Ex situ XRD patterns of PTCDA corresponding to the selected SOC points in (a). P stands for the pristine electrode; PW stands for a pristine electrode soaked in the saturated Mg(NO3)2 (aq) electrolyte, and GF stands for a glass-fiber separator. A scan of PW was obtained to show that some peaks arose due to the presence of Mg(NO3)2 residue in the dried electrode; such peaks are indicated by asterisks. Ex situ XRD patterns of PTCDA at the selected points in Fig 2a. demonstrate peak shifts in the 2θ region from 8° - 13° and ~ 27.5°.