Reports: ND955729-ND9: Strategies To Reduce Interfacial Viscosity in Nanoparticle-Based Enhanced Oil Recovery (EOR) Fluids
Surita R. Bhatia, PhD, State University of New York at Stony Brook
Nanoparticle and nanoparticle-polymer dispersions are emerging as promising candidates for fluids used in a number of Enhanced Oil Recovery (EOR) processes, including drilling and flooding. Rheological and interfacial properties of these fluids are known to be key to their use in EOR, and a great deal of fundamental research has been conducted on the bulk rheology and interfacial tension of nanoparticle-based dispersions. However, analysis of the flooding process suggests that an additional property, the interfacial viscosity, to be crucial in promoting oil recovery after displacement, with a low interfacial viscosity being desirable for EOR. Until recently, reliable measurement of interfacial rheology of nanoparticles at interfaces has been difficult. However, recent advances in instrumentation now allow for reproducible measurement of the rheology of nanoparticle-loaded interfaces.
Our goal for this project is to develop a fundamental understanding of how particle-particle interactions and particle-polymer interactions can be used to control the rheology of nanoparticles at air-water and air-brine interfaces. In doing so, we will test the extensibility of concepts from 3D (bulk) rheology of nanoparticles to 2D systems and will design new strategies to control the interfacial viscosity of nanoparticle dispersions. In Year 1 of the grant, we explored the interfacial rheology of the synthetic clay laponite, which is a disk-shaped nanoparticle with a diameter of 25 nm and a thickness of 0.92 nm with the empirical formula Na+0.7[(Si8Mg5.5Li0.3)O22(OH)4]-0.7, together with two water-soluble polymers, poly(ethylene oxide) (PEO) and poly(acrylic acid) (PAA) of varying molecular weights and concentrations.
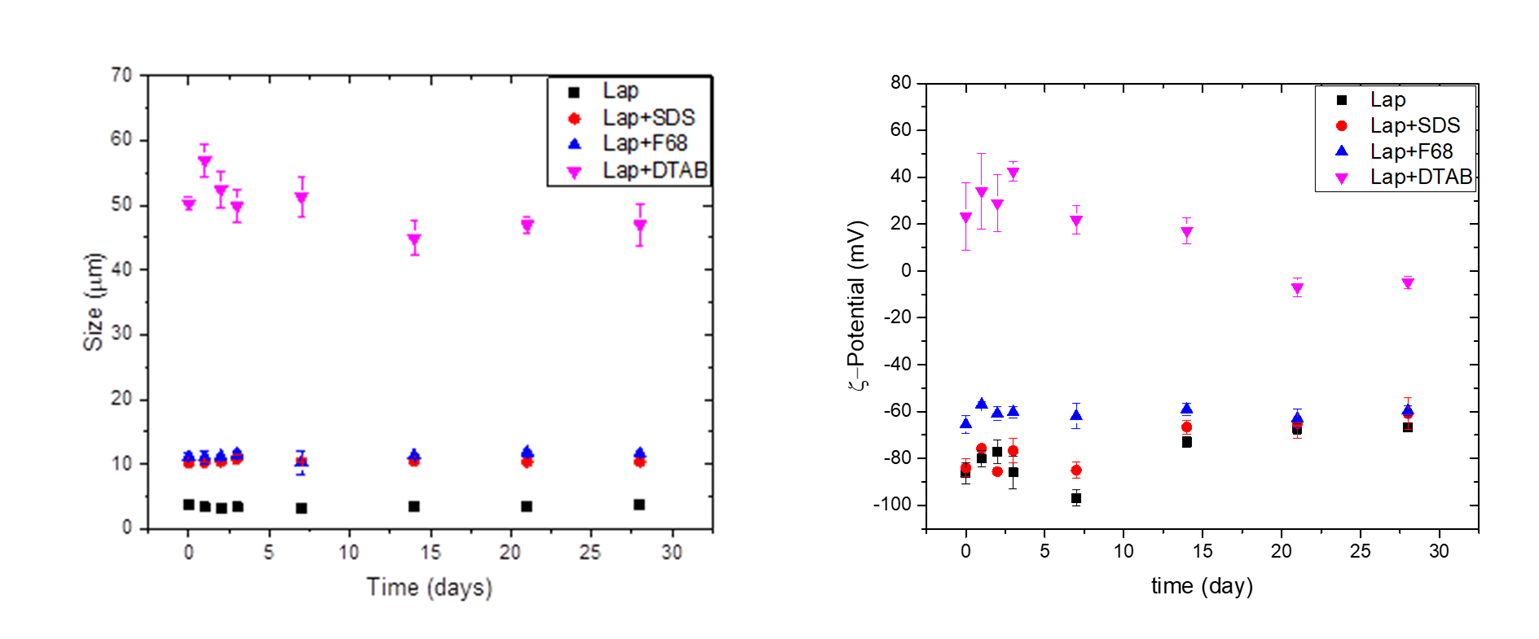
In Year 2 of the grant, we have applied the knowledge gained in Year 1 to explore the stability and structure of oil-in-water emulsions comprising laponite with and without surfactants (dodecyltrimethylammonium bromide (DTAB), Pluronic F68, and sodium dodecyl sulfate SDS). We investigated these systems using dynamic light scattering (DLS), zeta-potential, optical microscopy, and rheology. Our initial results show that the surfactants had a significant effect on the creaming behavior of the emulsions (Fig 1) and on the average droplet size and zeta-potential (Fig 2). The DLS and zeta-potential results show an average droplet size of 3 µm and a zeta-potential of -90 mV for the emulsions stabilized with only laponite. With the addition of surfactants, both the droplet size and zeta-potential increase, suggesting absorption of surfactants on the surface of laponite particle.
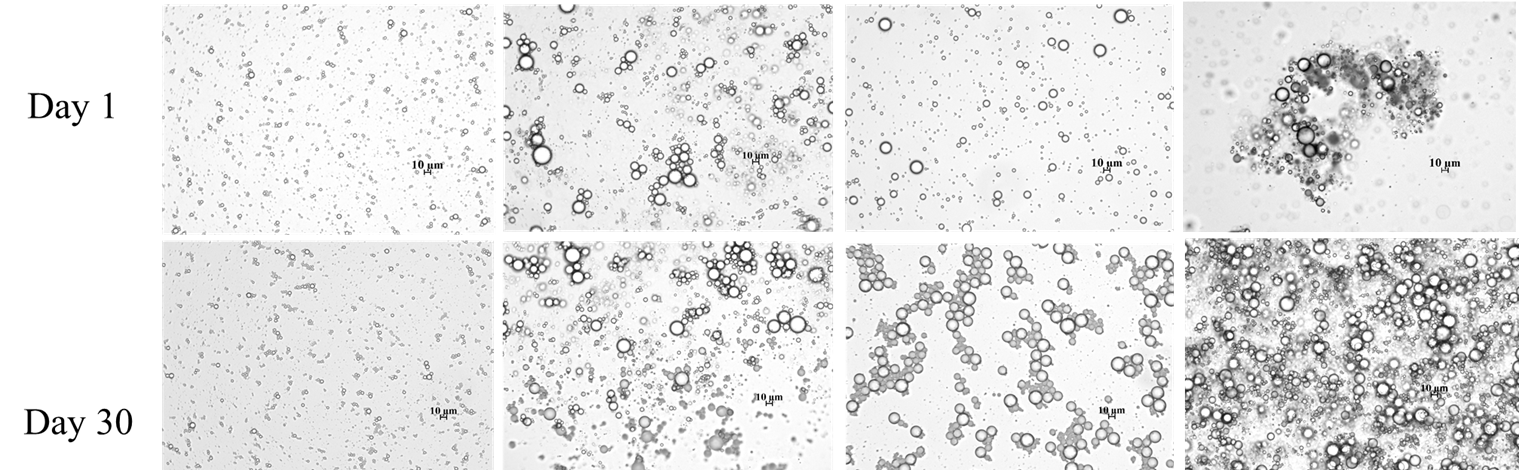
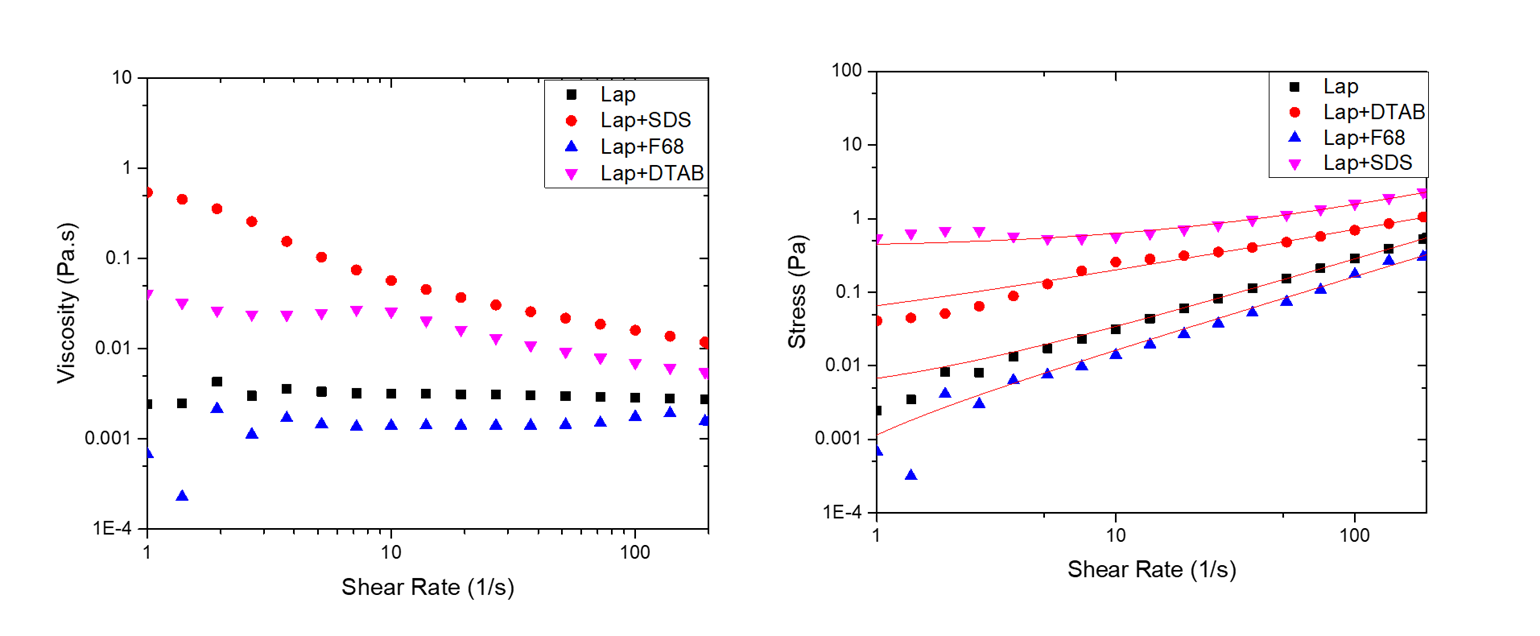
Our optical microscopy results (Fig 3) suggest that addition of surfactants leads to different types of larger-scale structure in the emulsions. With SDS, there is some evidence of formation of larger flocs, while addition of F68 shows formation of chain-like structures, possibility due to F68 bridging between emulsion droplets. Addition of DTAB leads to both large flocs and perhaps some network-like structure. The formation of large flocs observed in samples containing SDS and DTAB corresponded with a significant increase in the viscosity (Fig 4), while the nonionic surfactant F68 was not found to have a significant effect on the rheology.
In addition to these new experimental results, in Year 2 we wrote up and published some results from Year 1, and those results have appeared in the journal Colloids and Surfaces A (B. Zheng, S. R. Bhatia. “Cluster Formation During Aging of Colloid-Polymer Dispersions.” Colloids and Surfaces A 2017, 520, 729–735).
Figure 1. The effects of various surfactants on the stability and creaming behavior of laponite-stabilized emulsions.
Figure 2. The effect of various surfactants on the emulsion droplet size (left) and zeta-potential (right) for laponite-stabilized emulsions as a function of time.
Figure 3. Microscopic images of the laponite-stabilized emulsions without and with surfactant. From left to right: laponite, laponite + SDS, laponite + F68, and laponite + DTAB
Figure 4. Results from steady shear rheological studies on emulsions with and without added surfactant.