Reports: ND356705-ND3: Development of the Synthesis of Mixed Oxysulfidometalates and of a New, General Methodology for the Preparation of Terminal Disulfide Complexes
James P. Donahue, PHD, Tulane University
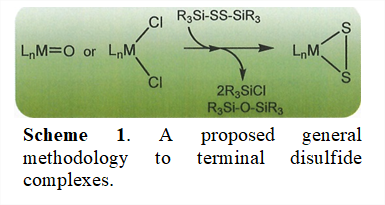
The key objective for this project is the development of a general, straightforward methodology for the synthesis of terminal disulfide complexes. The disulfide functional group is a subject of interest because it has been implicated, for example, as the catalytically essential moiety along reactive edges of MoS2. Lacking, however, are satisfactory general methods for preparing well-defined M(η2-S2) small molecules that might support catalytic activity under homogeneous conditions in which informative spectroscopic study would be feasible.
Our approach to this objective has been to assess the feasibility of using bis(trialkylsilyl)disulfide molecules as delivery agents for the disulfide ligand (Scheme 1). The lynchpin to the idea is that the relatively weak Si‒S can be exploited to deliver the S22- group to an appropriate LnMn complex while a much more thermodynamically favorable R3SiCl or (R3Si)2O byproduct can result from ligand exchange at metal. Among choices for bis(trialkylsilyl)disulfide molecules, our attention has been given to iPr3SiSSSiiPr3, in part because of the availability of iPr3SiSH. We have found, however, that a survey of the reactivity of iPr3SiSSSiiPr3 with a variety of transition metal chloride complexes leads to no observable reaction. These outcomes are attributed to kinetic inertness provided by the steric profile given by the multiple iPr groups.
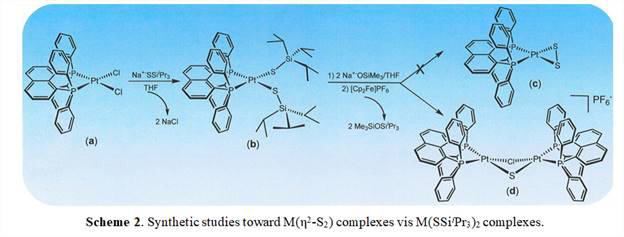
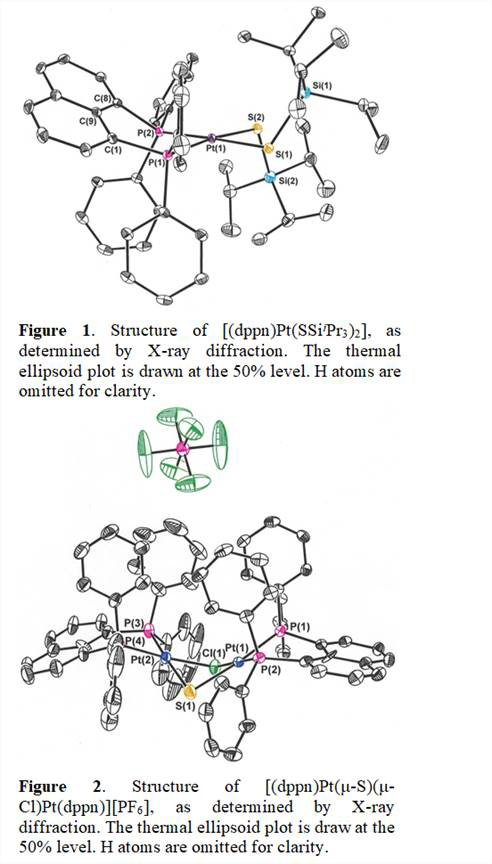
As an alternative to the reaction type illustrated in Scheme 1, we have studied the possibility of dissecting the reaction into parts, as illustrated in Scheme 2. The platinum complex illustrated has been selected for focused study because a variant of it has been shown to support a terminal disulfide ligand. We have recently demonstrated that displacement of Cl- from [Pt(dppn)Cl2] (dppn = 1,8-bis(diphenylphosphinonaphthalene)) by Na+-SSiiPr3 does indeed occur to afford [(dppn)Pt(SSiiPr3)2] ((b) in Scheme 2). This product molecule has been identified by X-ray crystallography (Figure 1). Further, we find that the -SSiiPr3 ligands in [(dppn)Pt(SSiiPr3)2] are, as intended, subject to further reactivity. Initial efforts to unmask the +SiiPr3 protecting groups and oxidize the resulting sulfide anion to disulfide made use of commercially available [nBu4N]+F- solutions. However, while reactions have certainly observed between [(dppn)Pt(SSiiPr3)2] and [nBu4N]+F-, the reactivity has not been clean and has not yielded the intended terminal disulfide ((c) in Scheme 2). This complication is, in hindsight, attributed to the fact that commercially sold solutions of [nBu4N]+F- in THF are typically ~5% H2O.
This supposition is supported by a recent observation that use of Na+[OSiMe3] as unmasking agent, although more bulky, is more clean in its reactivity and yields the di-platinum compound illustrated in Figure 2 and as (d) in Scheme 2. Mixed sulfide-chloride bridging ligands are inferred on the basis of net charge; Cl- is presumed to have originated from the use of CH2Cl2 in the recrystallization of this product or from residual NaCl from the preceding step.
In continuing work on development of a protocol for use of R3SiSSSiR3 as delivery agents for inorganic S22-, we are exploring several variants of the dppn ligand that differ in the steric profile that they project toward the other side of the metal ion. These variants include the 3,5-dimethylphenyl and the 2,4,6-trimethylphenyl derivatives. It is currently unclear how sensitive the [(phosphine)2Pt(η2-S2)] is toward ambient atmosphere and how much steric bulk is essential to stabilize it against a tendency to form (μ2-S)2 or (η2,η2,μ2-S2) species. If the reaction conditions and supporting ligand attributes can be identified which lead to isolation of [(phosphine)2Pt(η2-S2)], our studies will be extended toward gold, iridium, and mercury complexes because these elements appear most likely to support the intended ligand type.
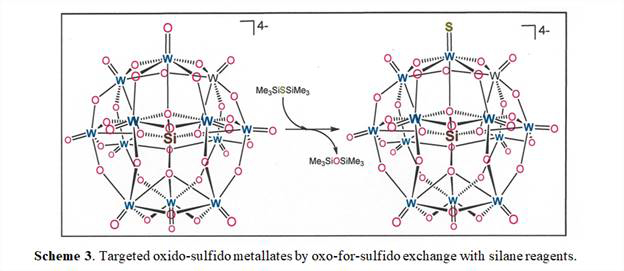
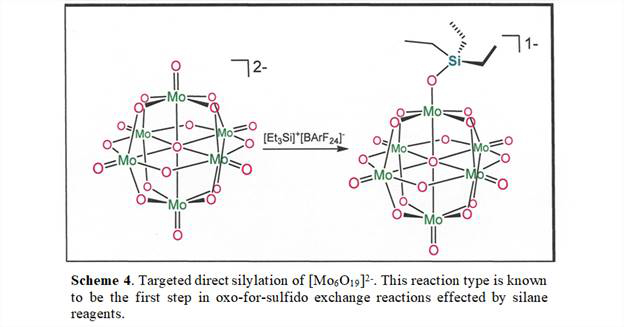
A second theme of research outlined in the original project proposal was an examination of ways by which sulfur might be incorporated into polyoxometallate anions. The general strategy is to modify well-defined polyoxometallates with silane reagents (Scheme 3), which have been demonstrated to be effective for oxo-to-silyloxide and oxo-to-sulfido transformations in mono-metallic systems. Initial efforts focused upon [W12SiO40]4-, but no decisive evidence has been developed indicating that exchange of terminal oxo ligand in [W12SiO40]4- is occurring. In hindsight, what may be complicating the assessment of possible products in Scheme 3 is the occurrence of multiple isomers of [W12SiO40]4-, which makes difficult the crystallization of samples well-suited for X-ray diffraction. In view of this apparent challenge, we are directing attention to [Mo6O19]2-, which is not subject to isomerism, and we are endeavoring to accomplish simple silylation of terminal oxo ligand with [Et3Si]+ according to Scheme 4. This reaction type is the first step in oxo-for-sulfido exchange, and such intermediates have been isolated in monometallic systems. Our current belief is that a reactive silylium cation, such as [Et3Si]+[B(C6H3-3,5-(CF3)2)4], should silylate a terminal oxo ligand in [Mo6O19]2- and sidestep any complicating undesired redox chemistry. The [Et3Si]+ cation has no capacity to reduce the [Mo6O19]2- cluster and instead can only leave it as fully oxidized, diamagnetic, and mono-negative, which will facilitate product identification by 1H NMR spectroscopy and mass spectrometry.
This research has motivated both the PI, James P. Donahue, and the primary student involved, Ms. Malathy Selvachandran, to first of all be mindful of the importance of orienting chemical research to applied ends and to find the ways to articulate a justification for fundamental chemistry in terms of an objective that is useful. The beginning of every presentation on this project has required the student to explain why it is that terminal disulfide complexes are important and why it is that there is value in devising more efficient ways toward them. Further, this research has brought focus to the need to be broadly read in the generally relevant area and to be innovative in finding alternative pathways to a synthetic target if one’s first choice does not work. For the PI, this project has been significant in that it is the first time he has undertaken and thought about a synthetic methodology problem as opposed to a targeted synthesis problem.