Reports: UR557053-UR5: A kinetic Study of the Oxidation of Chemisorbed Hydrocarbons by Non-Thermal Plasma
Juan G. Navea, PhD, Skidmore College
This project represents an alternative method for the oxidation and functionalization of hydrocarbons, where free radicals are generated through a radio frequency discharge in a non-thermal plasma. During the first year of the grant we built a plasma-surface reactor for the generation of O(3P) free radicals in a controlled environment. The system was then optimized to carry out reactions between O(3P) and surfaces coated with hydrocarbons. The developed system allows the free radicals to react with adsorbed hydrocarbons while a spectroscopic probe analyzes the chemical changes in situ. In the next pages we briefly summarize our progress on (1) construction and optimization of the reaction system and (2) heterogeneous reactions between O(3P) and chemisorbed benzaldehyde.
1. Construction and optimization of plasma reaction system.
a. Plasma reaction system
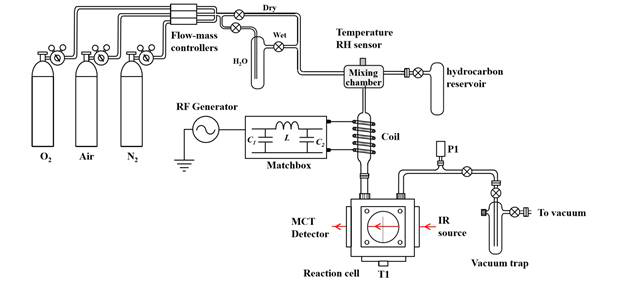
The first year of the project was dedicated to the construction and optimization of the state-of-the-art plasma reaction system. The reaction setup is described in Figure 1:
Figure 1: Diagram of the plasma system for reactions between oxygen non-thermal plasma and hydrocarbons adsorbed on alumina (Al2O3).
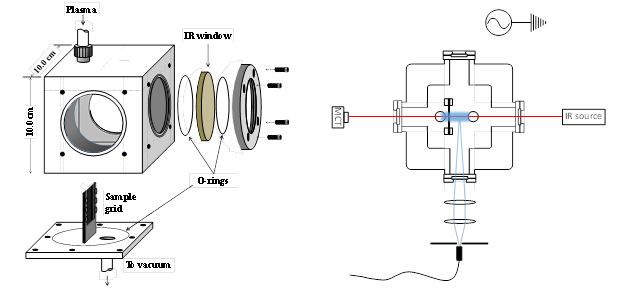
Oxygen plasma containing O(3P) is generated in the coil region via radio frequency (RF) discharge on a low oxygen pressure system (20 to 150 mtorr). The resulting plasma beam is directed to a reaction chamber where the O(3P) beam enters in contact with organic species chemisorbed on aluminum oxide (Al2O3). A custom-made radio frequency generator and coil (Manitou Systems Inc.) was used to generate 100 watts of radiofrequency discharge, enough to generate the plasma. The aluminum reaction chamber (T1) receives the plasma plumes and directs it towards the organic compound chemisorbed onto Al2O3. The reaction chamber, shown in Figure 2, consists of a custom-made aluminum cube that fits in the sample compartment of an FTIR spectrometer. The reaction chamber has a sample holder that consists of a 1.2×2.0 cm tungsten grid (20 μm wire diameter) that holds the sample and allows for transmission FTIR. The plasma inlet was designed so that the plasma would flow across the sample. As shown in Figure 2, two quartz windows on opposite sides of the reaction chamber allow for UV-Vis detection, and two barium fluoride (BaF2) windows allow for IR analysis.
Figure 2. Left: Diagram of the aluminum reaction chamber designed for plasma reactions. Right: detection system used that contained an IR source and windows, and an ocean optics spectrometer with quartz windows detecting the plasma emission lines (represented with blue lines).
b. Optimization of the plasma reaction system
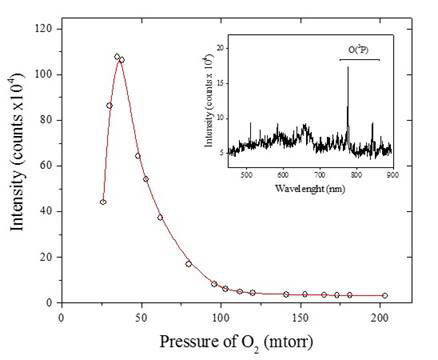
After construction of the reaction system, tests were performed to determine the optimum conditions to generate O(3P) in the reaction site. This optimization was performed by using a UV-Vis spectrometer equipped with a fiber optic (Ocean Optics). The O2 base pressure of the system was modified and the emission intensity for O(3P) at 777.06 nm, the ground state of neutral atomic oxygen, was used to determine the optimum pressure for O(3P) generation.
The pressure dependence optimization in Figure 3 shows an increase in the intensity of the O(3P) emission line as a function of pressure. For our experimental conditions, the maximum generation of (O3P) radicals takes place at a base pressure of O2 in the coil region of 50 mtorr.
Figure 3. The emission intensity as it relates to the pressure of oxygen gas within the reaction system. The insert shows the two lines corresponding to O(3P) resulting from a plasma discharge in 98 mtorr O2.
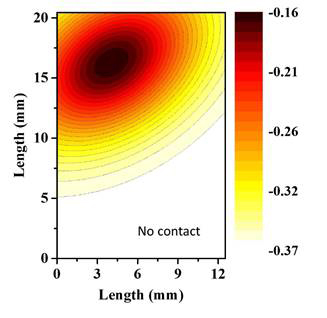
In order to optimize the reaction system, experiments were conducted by adsorbing 1,2-dyhydroxylbenzene onto Al2O3. The tungsten grid containing the coated Al2O3 was placed inside the reaction chamber under an approximately 1.0 Torr partial pressure of 1,2-dyhydroxylbenzene. After adsorption, the reaction chamber is placed under vacuum to remove any weakly adsorbed compound (physisorbed). The optimized plasma was then allowed to flow through the system. The resulting oxidation was investigated ex situ in an FTIR microscope in order to map the reaction site. Figure 4 shows a Gaussian gradient of oxidized product from the centre of the plasma-surface point of contact with the surface.
Figure 4 shows the point of direct contact between O(3P) and surface-bound organic compounds, which is the most sensitive point of analysis. IR beam used for in situ analysis of the heterogeneous reactions is, therefore, in the upper half of the tungsten grid containing the sample.
Figure 4. FTIR mapping of oxygen plasma on the surface. Plasma was generated under an oxygen pressure of 75 mTorr and allowed to react on a 1,2-dyhydroxylbenzene surface for 1 min.
2. Heterogeneous Reaction: Adsorbed Benzaldehyde + O(3P)
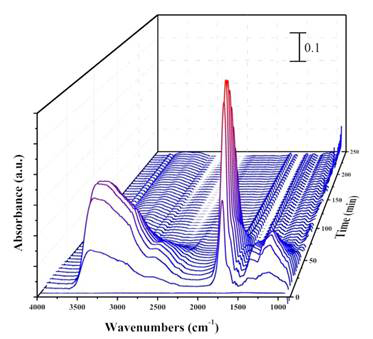
Infrared scans are collected as the reaction between plasma and adsorbed benzaldehyde takes place, with the infrared spectra referenced to C7H6O(a) on Al2O3. As the experiment progresses, new surface-bound products are observed upon plasma treatment. In Figure 5, the hydroxyl group absorption band (3250 cm-1) was observed to increase as a function of reaction time. There were also spectral changes observed that indicated a growth of carbonyl groups at 1740 cm-1.
Figure 5. The IR spectra of plasma oxidation of benzaldehyde, referenced to adsorbed benzaldehyde. The spectra were taken at time intervals of 35 seconds, showing oxidation with respect to the duration of plasma treatment.
The preliminary study showed an initial increase in the functionalization by oxygen containing groups up to 10 minutes after the reaction. As can be seen in Figure 5, after 10 minutes the vibrational bands of the oxidation products decrease, due to the removal of products by continued plasma treatment.
3. Year two objectives
Year two will concentrate on kinetic data acquisition of the reaction between O(3P) and chemisorbed hydrocarbons. Hydrocarbons used in Year 2 consist on several proxies with different numbers of instaurations, such as cyclohexane and cyclohexene. Our objective will be to obtain initial rates of surface-bound oxidation products. In addition, by capturing any gas-phase organics, desorbed products will be analyzed ex situ via GC-MS. Finally, computational simulation of the adsorbed reactants and products will be used as a first step in simulating the chemical reaction between adsorbed hydrocarbons and O(3P).