Reports: DNI555094-DNI5: In-Situ SERS Study of Au-Catalyzed Oxidation of Propene Using Multifunctional Microstructured Optical Fiber Platform
Fei Tian, PhD, Stevens Institute of Technology
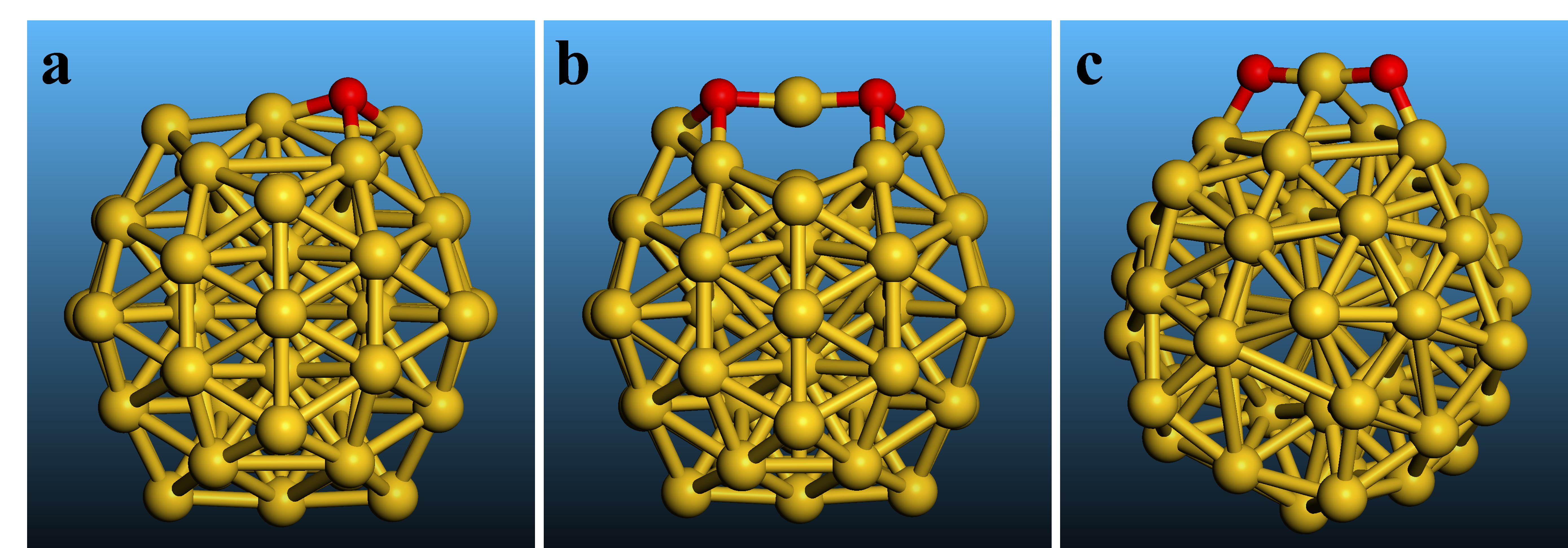
Studies of atomic oxygen adsorption on well-defined surfaces of Au single crystals identified two structures based on characterization with several techniques. Atomic oxygen was generated using decomposition of O3. The first structure with isolated O atoms was observed at low surface oxygen coverage (Figure 1a). The second structure, as a precursor of a surface oxide, was observed at higher coverage when two oxygen atoms push a neighboring Au atom from the surface and form a dimer with the elevated Au atom in between as Au2-O-Au-O-Au2, and with the O atoms in pseudo threefold sites (each O atom is bonded to three Au atoms) (Figure 1b). In this work, a third structure is identified on surfaces of Au nanoparticles. When O atoms do not need to push an Au atom from a flat surface, they form an Au-O-Au-O-Au structure with the O atoms in bridge sites (each O atom is bonded to two Au atoms) (Figure 1c).
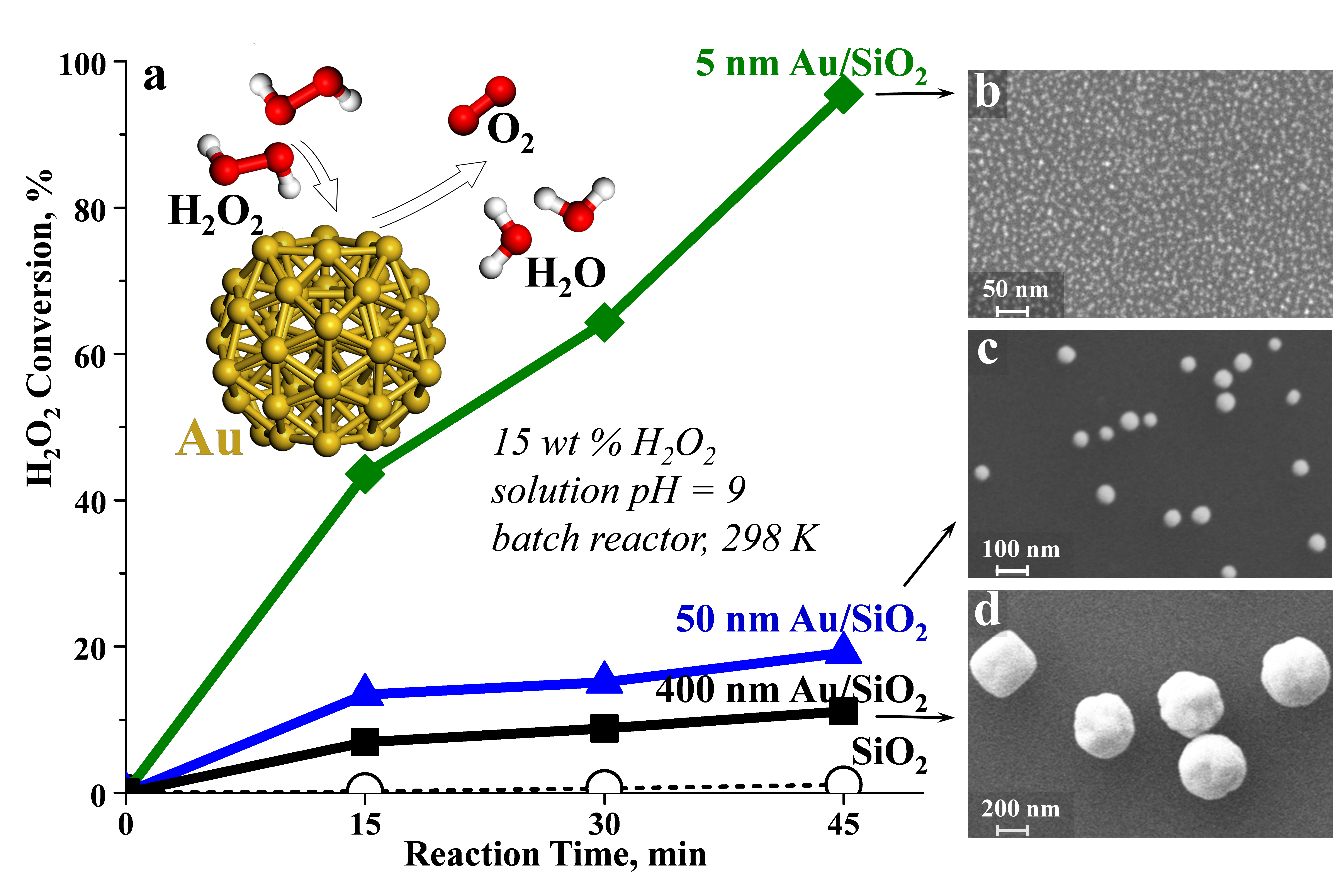
Catalysts with 5, 50 and 400 nm monodisperse Au particles supported on SiO2 (Figures 2b-d) were prepared using colloidal solutions, in which the Au nanoparticles were stabilized with citrate organic ligands. The total Au surface area was also constant in all experiments. Catalytic decomposition of a 15 wt % aqueous H2O2 solution to H2O and gas-phase O2 over the clean Au nanoparticles was monitored with in situ SERS measurements for detecting reaction intermediates on Au and with titration measurements for evaluating the time-dependent extent of H2O2 conversion. The reaction rate of H2O2 decomposition decreased with increasing Au particle size (Figure 2a).
Time evolution of in situ Raman spectra for the 400 nm Au/SiO2 at pH=9 is compared in Figure 3a to a spectrum of the aqueous H2O2 solution collected in a separate experiment without Au under the same conditions. In addition to a broad Raman band at 940 cm-1 for SiO2, the spectrum for liquid-phase H2O2 without Au exhibited a single band at 875 cm-1 in the analysed wavenumber range of 550-1200 cm-1. Immediately after adding H2O2 to the 400 nm Au/SiO2 (reaction time of zero in Figure 3a), essentially the same spectrum with a single band at 875 cm-1 was observed.
After the start of H2O2 decomposition, two new Raman bands at 590 and 822 cm-1 were observed (Figure 3a). The band at 822 cm-1 is assigned to OOH surface species. The band at 590 cm-1 is assigned to the Au-O-Au-O-Au atomic oxygen structure (Figure 3b) based on an agreement with the calculated frequencies of 546-558 cm-1 (Structure D in Table 1). This assignment is discussed below
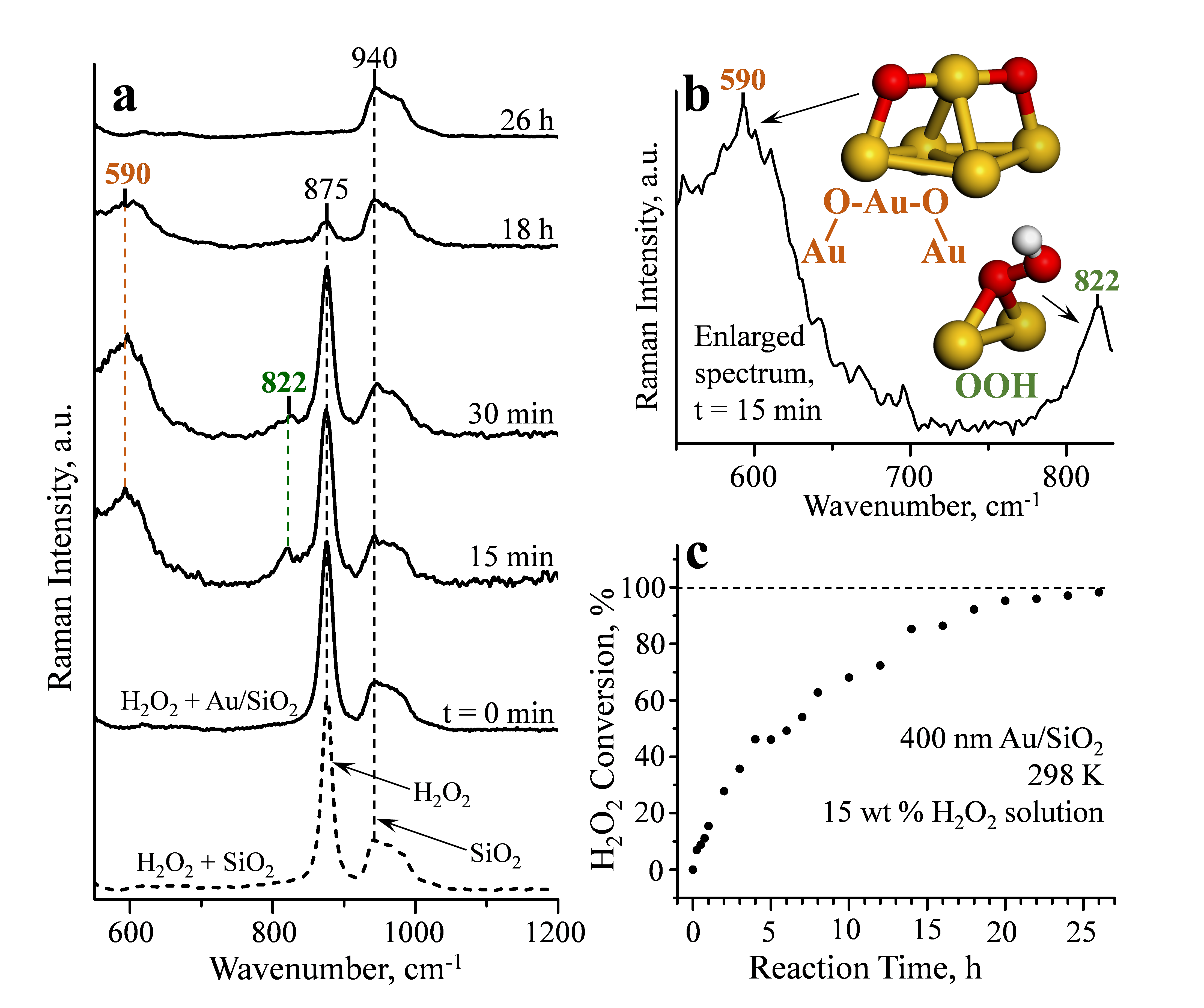
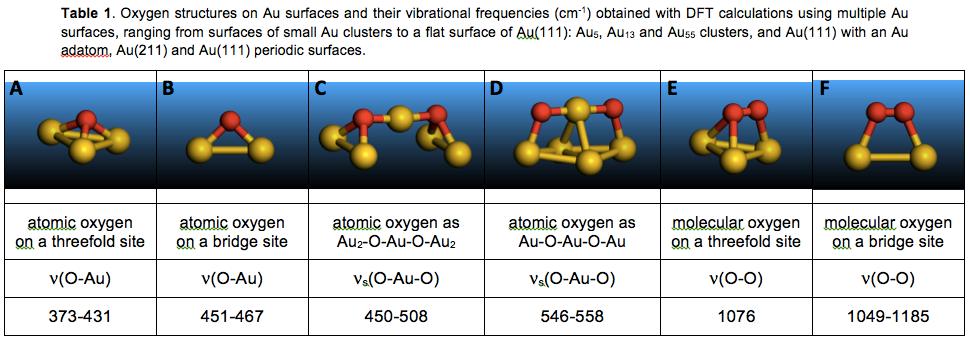
In order to assign the experimental Raman band at 590 cm-1, all previously spectroscopically identified oxygen structures on Au surfaces were considered and evaluated with DFT calculations. The calculated vibrational frequencies are summarized in Table 1. Depending on the Au particle size, this frequency for an O atom adsorbed on a flat surface that resembles Au(111) (Figure 1a) varies from 373 to 431 cm-1 (Structure A in Table 1). For Au surfaces with coordinatively unsaturated Au atoms, adsorption of an isolated O atom becomes energetically preferable on a bridge, instead of a threefold, site with ν(Au-O) of 451-467 cm-1 (Structure B in Table 1). However, the Raman band at 590 cm-1 is outside of the range of all calculated frequencies for isolated oxygen atoms in Table 1 (Structures A and B) and the reported experimental values and, therefore, this assignment can be ruled out.
For molecular oxygen, the calculations suggest that adsorption on Au(111) is metastable with a preferred threefold site and ν(O-O) of 1076 cm-1 (Structure E in Table 1). On the evaluated non-flat surfaces, the preferred adsorption site is a bridge (Structure F in Table 1). Importantly, however, the Raman band at 590 cm-1 is outside of the range of all calculated frequencies for molecular oxygen in Table 1 (Structures E and F) and the reported experimental values and, therefore, this assignment can be ruled out. Since both simple atomic and molecular oxygen surface species can be ruled out, the 590 cm-1 band must be due to a more complex structure.
When neighbouring O atoms do not need to push an Au from the surface, another atomic oxygen structure forms (Structure D in Table 1, Figure 1c). In this Au-O-Au-O-Au structure, two O atoms preferentially bind to and share a coordinatively unsaturated Au atom with an Au-O bond distance of 0.194-0.196 nm. In addition, each O atom binds to another surface Au atom with a larger bond distance of 0.203-0.213 nm. Both O atoms are, thus, in bridge sites, as opposed to threefold Au sites on Au(111). The calculated frequencies for νs(O-Au-O) are 546-558 cm-1 (Structure D in Table 1), close to the position of the 590 cm-1 Raman band. Furthermore, coordinatively unsaturated Au atoms must be serving as active catalytic sites for H2O2 decomposition because the decomposition rate decreases significantly with increasing Au particle size (Figure 2), as the surface of these nanoparticles becomes progressively flatter, resembling Au(111). Therefore, the Raman band at 590 cm-1 can be assigned to the newly identified Au-O-Au-O-Au structure. The observation of this atomic oxygen structure in H2O2 decomposition provides the first direct evidence that the reaction mechanism proceeds through splitting of all O-O and O-H bonds, not just through the formation of OOH and OH surface species, which will advance the development of improved catalyst formulations for the direct H2O2 synthesis.











