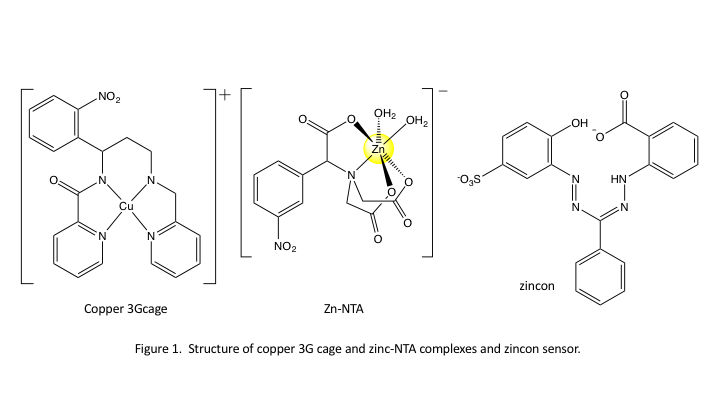
Reports: ND655242-ND6: Developing a Metal-Ion Burst Technique to Explore Ionic Strength Jump Dynamics
Nancy E. Levinger, Colorado State University
Debbie Crans, Colorado State University
The overarching goal for this project has been to create a photolytic method to release metal cations on an ultrashort timescale permitting us to explore the very first metal ion binding by proteins associated with neurodegenerative diseases, such as Alzheimers. We applied ultrafast laser spectroscopy on two model metal cage complexes to explore the development of this method. We compare and contrast results for experiments in bulk aqueous solution and in the confined environment in reverse micelles.
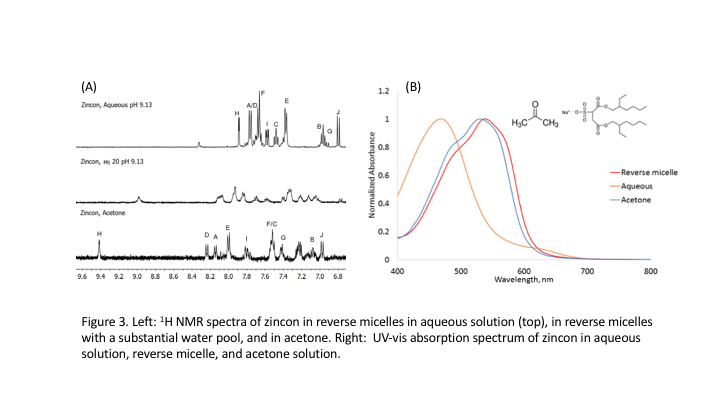
The differences in zincon absorption spectra in aqueous and RMs could indicate
that zincon partitions into the RM interface. We utilized
1D and 2D NMR spectroscopy to explore the origin of zincon
spectral changes. Fig. 3. 1D 1H NMR of free zincon
and Zn-zincon in bulk aqueous solution and in RMs
show significant differences in peak positions and spectral bandwidth
suggesting that zincon may embed in the interface.
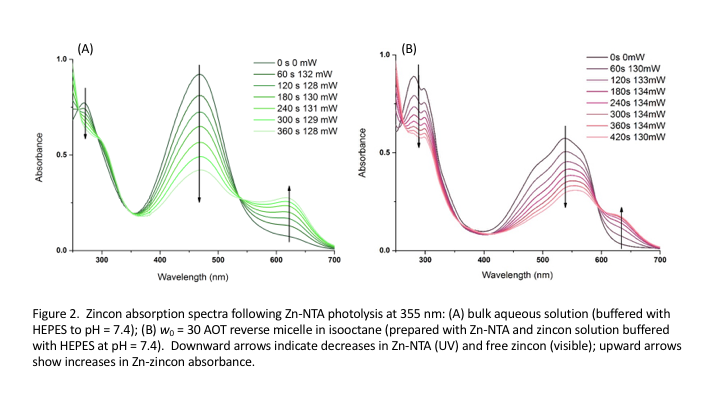
We have shown that we can
successfully photolyze the metal-cage complexes in
aqueous solution and within reverse micelles. Photolysis in reverse micelles resembles
the dynamics in aqueous solution. We are working to explain the differences in
the two systems, why we think those differences exist and how they relate observed
zincon dynamics in the two systems. To our knowledge,
the metal-ion burst within reverse micelles before has not been measured. This
could have biological implications of membrane associated proteins, small
molecules and other structures which use metal ions or metal complexes which
must diffuse through an ionic layer before finding their mate to either chelate
the metal ion or exchange their metals with an awaiting component in a
membrane.
Impact on student
education: The
research reported here has served to train students with laboratory and
critical thinking skills. Dr. Richard Cole, in the Levinger group has led
time-resolved spectroscopy studies, training graduate student Cheryle Beuning and several
undergraduate students. Ms. Beuning has worked
closely with undergraduate students who presented 3 posters at the CSU
Celebrate Undergraduate Research and Creativity poster session, spring 2017. Ms.
Beuning presented results at the 2017 spring national
ACS meeting.5 Two undergraduate students will co-author the
manuscript Ms. Beuning is currently writing about
project results.
References 1. Ciesienski, K. L.; Franz, K. J., 2011, Keys for Unlocking Photolabile Metal-Containing Cages. Angew.
Chem.-Int. Edit. 50, 814-824. 2. Basa, P. N.; Antala, S.; Dempski, R. E.; Burdette, S. C., 2015, A Zinc(II) Photocage Based on a Decarboxylation Metal Ion Release
Mechanism for Investigating Homeostasis and Biological Signaling. Angew. Chem.-Int. Edit. 54, 13027-13031. 3. Platte,
J. A.; Marcy, V. M., 1959, Photometric determination of zinc with zincon - application to water containing heavy metals.
Anal. Chem. 31, 1226-1228. 4. Sabel, C. E.; Neureuther, J. M.; Siemann,
S., 2010, A spectrophotometric method for the determination of zinc, copper,
and cobalt ions in metalloproteins using Zincon. Anal. Biochem. 397,
218-226. 5. Beuning, C.; Paryani, T.;
Barkley, N.; Basa, P.; Cole, R.; Levinger, N.;
Burdette, S.; Crans, D., 2017, Photolysis of a
Zn(II)-nitriliotriacetate cage in AOT/isooctane
reverse micelles. Abstr. Pap. Am. Chem. Soc. 253, 1.