Reports: DNI756501-DNI7: Organocatalyzed Atom Transfer Radical Polymerization of Petroleum-Relevant Monomers Using Perylene Dyes as Visible-Light Photocatalysts
Garret Miyake, PhD, Colorado State University
The central objective of this PRF-supported research is to gain a fundamental understanding of organocatalyzed atom transfer radical polymerization (O-ATRP) to allow for the rational design of organic photoredox catalysts (PCs) that can polymerize petroleum derived monomer substrates to well-defined polymers of target molecular weights (MWs) and low dispersities (Ɖ). Continued work with perylene (PC 1) has enhanced our understanding of the O-ATRP mechanism allowing us to improve the efficiency of 1, and guide the design of other families of O-ATRP PCs.
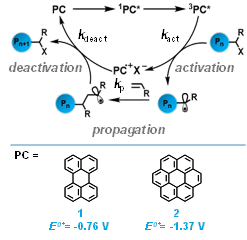
Figure 1. The proposed mechanism of O-ATRP (top) and structures and excited state reduction potentials of perylene (1) and coronene (2) are shown (bottom).
Critical to our progress in PC design has been an improved understanding of the O-ATRP mechanism (Figure 1). The proposed O-ATRP mechanism proceeds through photoexcitation of the ground state PC into a singlet excited state (1PC*) followed by intersystem crossing (ISC) to reach a longer-lived triplet excited state (3PC*). The 3PC* reduces the alkyl halide bond of the polymer chain end (possessing a reduction potential of ~ -0.6 V to -0.8 V vs SCE [saturated calmomel electrode]) to liberate a carbon centered radical. Polymerization proceeds via reaction of the polymer radical with monomers while the oxidized form of the PC (2PC•+) pairs with a halide ion to form PC•+X-. Oxidation of the radical chain end group by PC•+X- returns the polymer chain to a dormant state and the PC back to the ground state, completing the catalytic cycle. As with traditional ATRP, a rapid rate of deactivation is necessary to reduce the concentration of radical species in solution which minimizes bimolecular termination pathways, allowing for the synthesis of well-defined polymers.
Our ongoing work in this field and analysis of the O-ATRP mechanism has revealed several key photophysical and chemical PC properties, these include: 1. Efficient photon absorption [i.e. a high molar extinction coefficient (ε)], 2. High quantum yield (φ) of the excited state species, 3. Sufficient excited state lifetime (τ) for electron transfer reactions, 4. Strong excited state reduction potential [E⁰(PC•+/3PC*)], 5. Stable and sufficiently oxidizing 2PC•+ for deactivation [E⁰(PC•+/PC)], and 6. Efficient pairing of 2PC•+ with a halide ion to form PC•+X-.
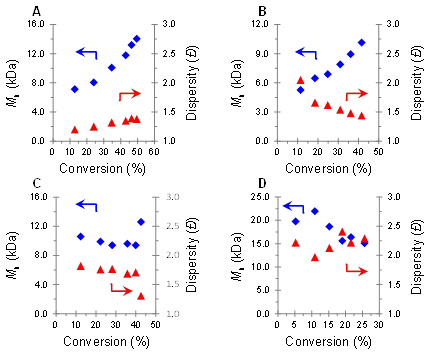
The first specific objective of this work is elucidation of the O-ATRP mechanism. Initial work using PC 1 for O-ATRP demonstrated its ability to polymerize petroleum-derived methacrylate, acrylate and styrenic monomers with excellent temporal control in pulsed irradiation experiments, but uncontrolled growth of polymer MW. For 1, we calculate that the excited state reduction potential of the 3PC* is barely strong enough to reduce the alkyl halide bond of the polymer chain end (-0.76 V vs SCE). In addition, 1 has a φ(triplet) of ~0.02 and a relatively short-lived τ of 4.9 ns, especially considering that efficient electron transfer processes typically require τ of the excited state species to be ~10 ns. Based on our proposed mechanism, the combination of these properties is predicted to cause inefficient formation of 3PC* and inefficient formation of PC•+X-. We envisioned that increasing the concentrations of 3PC* and PC•+X- via increased catalyst loading or more intense irradiation would improve polymerization results and lend credence to the proposed mechanism. Indeed, increasing the loading of 1 from 0.1 to 0.4 mol % for the O-ATRP of methyl methacrylate lowers the Ɖ of the resulting polymeric material from 1.82 to 1.38 despite a small decrease in initiator efficiency. These results were obtained using a more optimized irradiation apparatus which also resulted in increasing growth of polymer MW as a function of conversion (Figure 2A). The effect of light intensity on polymerizations with 1 was further investigated using 0.4 mol% catalyst loading (Figure 2A-D). As predicted, decreasing the light intensity led to loss of control over the polymerization which is indicated by an increase in polymer Ɖ and decrease in I*. These results are corroborated by improved performance of PC 1 when the polymerization is carried out in a flow reactor which is designed for more uniform lighting of reaction media.
Figure 2. Plots of Mn (blue diamonds) and Đ (red triangles) as a function of monomer conversion at 100% (A), 50% (B), 25% (C), 5% (D) relative irradiation intensity for the polymerization of methyl methacrylate mediated by PC 1.
To understand the relationship between the excited state reduction potential of organic PCs and their efficiency in O-ATRP, we sought to compare the performance of 1 to an organic dye with different redox properties. Coronene (2) was a suitable candidate for this investigation due to its structural similarity to 1 and higher excited state reduction potential (-1.37 V vs SCE). Compared to PC 1, O-ATRP with PC 2 is able to reach higher monomer conversions and produce polymer product with lower Ɖ (as low as 1.04), albeit with lower I*. These results provide preliminary evidence that PCs with stronger excited state reduction potentials are more efficient for O-ATRP and warrants further investigation.
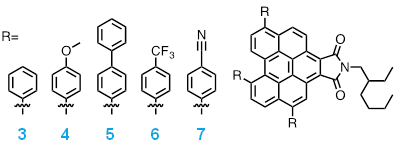
The second specific objective of this work is synthesis and application in polymerization of functionalized perylene dyes. A series of benzo[ghi]perylene imides (Figure 3) have been synthesized and characterized. Functionalization of the core with electron donating or electron withdrawing groups tailors the redox properties of parent compound 3 so that the effects of the excited state reduction potential of 3PC*and oxidation potential of 2PC•+ on catalytic performance can be studied further. Future work will apply these derivatives as PCs for O-ATRP to determine structure-property relationships for the design of superior PCs. Overall, support from the PRF has enabled deeper understanding of the O-ATRP mechanism and discovery of new O-ATRP PC families.
Figure 3. Structures of benzo[ghi]perylene imide derivatives.