Reports: UR754873-UR7: Surface Modification of Polyaniline Films and Nanofibers with Polymer Brushes
Timothy Hanks, Furman University
Introduction
In our year 1 report, we describe the synthesis of polyaniline (PANI) nanorods and their surface derivatization with thiol-terminated polyethylene glycol. These materials have now been fully characterized and a manuscript has been submitted for publication.
We also described the grafting of an atom transfer radical polymerization (ATRP) initiator on the surface of polypyrrole films. Preliminary experiments at that time demonstrated that the ATRP reaction could be successfully conducted on the surface attached initiators leading to a polyzwitterion being grafted from the film surface, much like the reaction conducted on polycrystalline gold. These studies are continuing as we try to improve reproducibility and to quantify the rates of reaction. However, we have demonstrated a key goal of our proposal, which was to show that the presences of the conducting polymer did not result in inhibition of the ATRP reaction.
Preparation of PEDOT and PPy Nanoparticles
 |  |  |  | ||||
In order to expand our investigation, we have prepared nanoparticles of poly(3,4-ethylenedioxythiophene)(PEDOT) and polypyrrole (PPy). Using previously reported procedures, both nanoparticles and elongated nanorods were selectively prepared (Fig. 1).
Figure 1. SEM images of PEDOT a) PEDOT nanoparticles b) PEDOT nanorods c) PPy nanoparticles d) PPy nanorods.
Preparation of thiol-terminated polyzwitterions
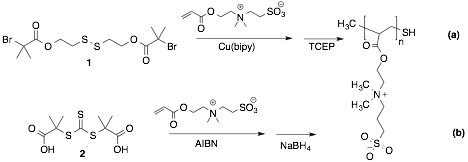
In our last report, we demonstrated that thiol-terminated polyethylene glycol purchased from commercial houses could be grafted onto PANI nanoparticles, increasing their water-dispensability dramatically. We have now prepared thiol-terminated poly(sulfobetaine metacrylate)(polySBMA) by controlled radical polymerization reactions (Fig 2). Pathway (a) shows the conditions for an atom transfer radical polymerization (ATRP) where a disulfide initiator (1) reacts with a copper catalysts to abstract a bromine atom and create a reactive carbon radical. Similarly, a reversible addition-fragmentation polymerization (RAFT, pathway (b)) was achieved by heating of the methacrylate monomer and the radical initiator AIBN in the presence of the RAFT reaction controlling agent 2. In each case, the reactions were run in either water or a water ethanol mixture. The rates of reaction were moderated by an equilibrium between active and inactive radicals in solution, which nearly eliminate side or termination reactions. In each case, the reaction required further treatment with the appropriate reducing agent in order to free the radical-sensitive thiol. The products were characterized by NMR, IR, thermal gravimetric analysis (TGA) and dynamic light scattering.
Figure 2. Mechanisms for living radical polymerization reactions used to make thiol-terminated poly(sulfobetine methacrylate). a) ATRP reaction, b) RAFT reaction.
Grafting to Conducting Polymer Nanoparticles
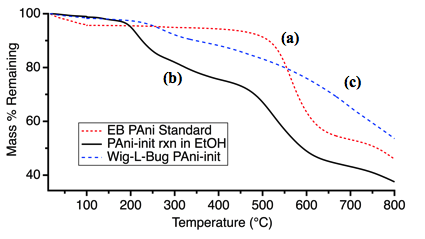
The thiol-terminated poly(zwitterions) are capable of effectively reacting with PANI and PPy nanoparticles to give highly water dispersible materials. Attempts to derivatize PEDOT nanoparticles have given ambiguous results to this point. Figure 3 shows the TGA data for poly(zwitterion)-derivatized PANI nanoparticles prepared in solution or through a solid-state grinding reaction verse an unreacted nanoparticle control. Our initial results indicate that depending on the reaction conditions and the molecular weight of the thiol, the mass attributed to the zwitterionic brush component of the resulting composites ranges from 5-30%. Additional characterization techniques are currently being employed to further characterize the products and to better quantify the impact of various reaction parameters. These include, NMR, IR, UV-vis spectroscopies, elemental analysis, DLS, contact angle goniometry and electrochemistry.
Figure 3. TGA data showing the mass loss of (a) PANI nanorod control, (b) poly(zwitterion) reaction in water and (c) poly(zwitterion) solid-state grinding reaction.
Grafting from Conducting Polymer Nanoparticles:
In our last report, we showed that ATRP reactions on PPy thin films took place at rates comparable to those of the same reaction on a gold surface. We have performed a similar process on PANI and PPy nanorods. While preliminary, these results indicate that efficient polymerization of poly(SBMA) from surface-anchored ATRP initiators on the nanorods also occurs. Studies are underway to better characterize the density of the initiators, the rates of polymer brush growth on the particles as compared to solution and the mass of zwitterion we are able to deposit on the surface as compared to the “graft-to” method.