Reports: ND356128-ND3: Cheap and Efficient Cerium Photosensitizers: Toward Excited State Metalloradicals for New Reactivity
Eric J. Schelter, PhD, University of Pennsylvania
The application of light as the ultimate atom-efficient reagent for organic transformations relies on the ability to photochemically produce radical intermediates, especially reactive carbon-based radicals. It is well-established that the scission of C-X bonds (X = Cl, Br, I) in organic substrates can be directly achieved under photolysis conditions to yield the corresponding radicals. However, the direct ¹-¹* excitation of aryl halides requires high energy and carcinogenic ultraviolet C (UVC) light (100-280 nm), which is only a small portion of solar spectrum. Alternatively, lower energy ultraviolet A (UVA light or black light, 315-400 nm) and visible light can be converted into chemical reduction potential using molecular photosensitizers to mediate single electron reduction reactions of aryl iodides and bromides. For example, photo-excited fac-Ir(ppy)3 (ppy = 2,2'-phenylpyridine) can reduce unactivated aryl iodides, and luminescent dinuclear gold(I) and copper(I) complexes were applied as triplet photoreductants for aryl bromide substrates under UVA light.
Our group has been at the forefront of developing lanthanide cations as effective photoredox mediators. For example, cerium(III) cations, with their single 4f electron, have simple, well-defined electronic structures featuring 2F5/2 ground states. The luminescence of Ce3+ cations originates from 5d-4f transitions, distinctly different than other Ln3+ cations where 4f-4f electronic transitions underlie their optical properties. The hypothesis of this project is that the excited state configuration for cerium(III) complexes, 5d1, can promote electron transfer to form cerium(IV) compounds and reduced organic products. Recently, we reported the first use of luminescent cerium(III) complexes as molecular photosensitizers to promote C-C bond formation reactions through visible light excitation. In initial work, we established that the reduction of substrates containing C-X (X = Cl, Br, I) bonds by cerium(III) complexes in their photo-excited state occurred through both inner- and outer-sphere single electron transfer (SET) pathways, taking advantage of both the reducing 2D excited state and the Lewis acidity of cerium(III) cations. These results encouraged us to expand the scope and knowledge of cerium(III) photosensitizers and translate them into efficient photoredox catalysts.
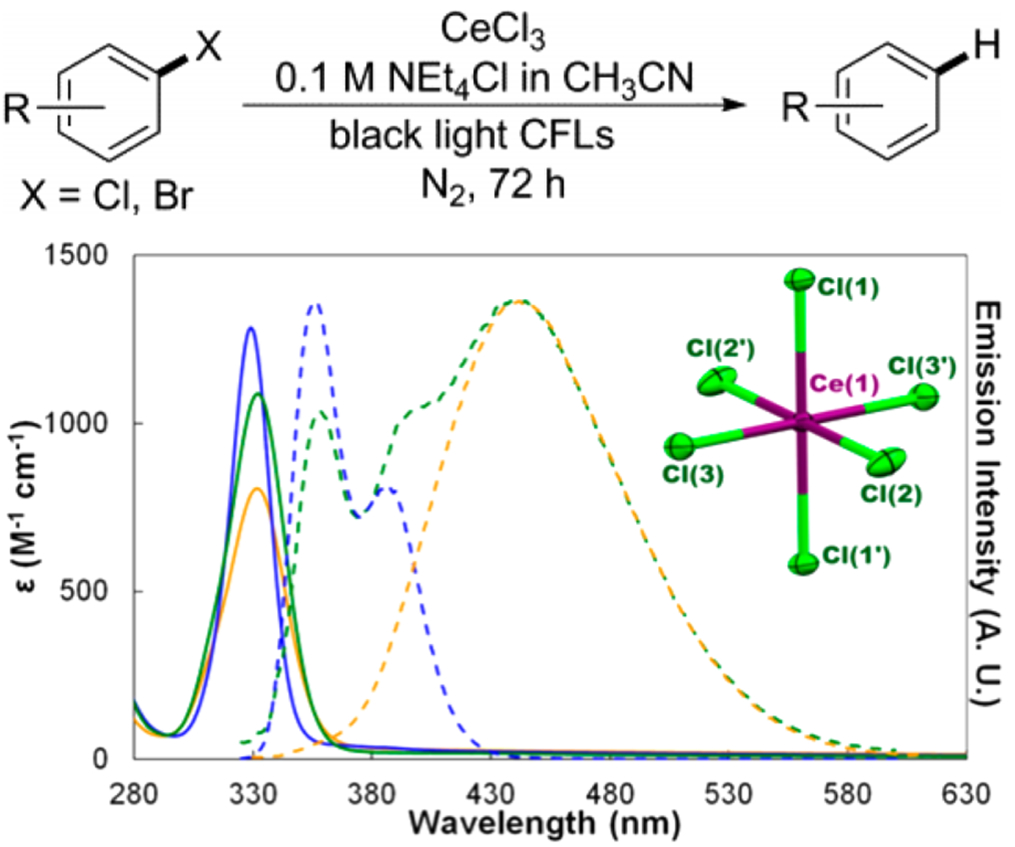
A central goal of the current New Directions project is the development and application of simple and easily prepared cerium photosensitizers. We have been studying the hexachlorocerate(III) anion, [CeIIICl6]3- (Figure 1.) We demonstrated the hexachlorocerate(III) anion was a potent photoreductant in acetonitrile solution with an estimated excited-state reduction potential of -3.45 V versus Cp2Fe0/+. This simple octahedral anion exhibited a lifetime of 22.1(1) ns, a photoluminescence quantum yield of 0.61(4) and fast quenching kinetics toward organohalogens. These results were reported in a JACS article on this system (see citation).
Aryl chlorides are ubiquitous compounds and are more prevalent than aryl bromides or aryl iodides. However, due to their thermodynamic potential required for their reduction, unactivated aryl chlorides are largely not accessible substrates in photoredox transformations with traditional photosensitizers. For the current project, we discovered that [CeIIICl6]3- was useful in this context, allowing for its application in the photocatalytic reduction of a range of unactivated aryl chlorides.
Notably, the [CeIIICl6]3- species was readily accessed by dissolving CeCl3 in CH3CN in the presence of excess NEt4Cl. Excess NEt4Cl was required due to a solution equilibrium with the Ce2Cl93- anion. The availability of both CeCl3 and NEt4Cl salts afforded a simple and inexpensive protocol for applying [CeIIICl6]3- anions as photoreductants. Despite a relatively short lifetime of the 2T2g* excited state of [CeIIICl6]3- of only 22 ns, the low excited-state reduction potential and especially the fast quenching kinetics of [CeIIICl6]3- enabled the dehalogenation reactions of Ar-X (X = Cl, Br, I). We expect the use of CeCl3 photochemically in one electron reactions will provide a more potent and readily available f-block element reducing agent than SmI2. It is also noteworthy that the structure and luminescence properties of molecular [CeIIICl6]3- species are strongly reminiscent of Ce3+ doped elapsolite and LaCl3 solid materials that are used as scintillators in radioluminescence applications. We expect that inspiration from existing bulk materials will benefit the development of molecular cerium(III) photosensitizers.
Figure 1. Photochemical dehalogenation reaction of aryl halides by the hexachlorocerate(III) anion (top). Absorption spectra (solid lines) and emission spectra (dashed lines) for bona fide CeCl63- (blue traces), a mixture of CeCl63- and Ce2Cl93- (green traces) obtained upon dissolution of pure [Et4N]3[CeCl6], and pure [Et4N]3[Ce2Cl9] (yellow traces).
In summary, we have investigated the unique structure and photophysical, photochemical properties of cerium(III) photosensitizers. The knowledge obtained from these studies will in term aid our design of new cerium(III) photosensitizers in, for example, photoluminescent materials with targeted properties and unknown photoredox catalysis. Support from ACS PRF for this project has launched this completely new research direction in my group. And we have incorporated ideas from the project into parallel ones involving separations chemistry. One undergraduate (Jerold Hertzog) was involved in the project during a summer REU research experience and earned co-authorship on a publication. He subsequently enrolled in graduate school at the University of Chicago. Four graduate students have benefitted from support of the project and two have graduated. One of the graduates is now a postdoc at Caltech and the other has started in a tenure track faculty position at a PUI.