Reports: DNI1055036-DNI10: Fundamental Investigation of Inorganic Metal Borophosphides
Kirill Kovnir, PhD, University of California, Davis
At the first stage of the project, efficient methods of synthesizing cubic BP were thoroughly investigated to gain understanding of the formation of the B-P bond. Synthesis techniques varied from traditional solid-state methods to using a Sn flux and finally to a novel solid-state metathesis technique. Variations of the temperature or time of annealing during metathesis reaction controlled the crystallinity of the produced BP. The compound clearly formed at temperatures as low as 700°C with only one day of annealing. Compared to the >1000°C required for traditional solid-state synthesis, metathesis is an energy-efficient method which proved to be successful in producing a compound that consists of elements that have widely different reactivities.
Using a Sn flux resulted in the discovery of one of the target compound, ternary tin borophopshide. Sn was found to substitute a part of B atoms in the crystal structure of BP. The shift in unit cell was detected by high resolution XRD data collected at the 11-BM beamline at the Argonne National Lab Advanced Photon Source. Sn-doping was additionally established by energy dispersive X-ray spectroscopy. This Sn-BP compound served as indication that new metal borophosphides are possible and are just waiting to be discovered. Other metal fluxes may be used to produce metal doped BP.
There are currently less than 10 known metal borophosphides since the first reported compound almost 50 years ago. Therefore, we aim to develop a methodology to synthesize these elusive compounds and study their properties. Previous attempts using traditional solid state or flux methods resulted in binary metal phosphides or borides. However, solid-state metathesis has been shown to be effective in producing difficult to synthesize or metastable compounds. For example, a series of new carbodiimides, with anions that are isostructural to the anions in some existing metal borophosphides, have been synthesized using this technique. As such, we began with synthesis of Na3BP2 as a starting material in preparation for metathesis reactions. Our hypothesis was that the B-P covalent bond in Na3BP2 would be conserved during the reaction with a transition metal halide to form a new metal borophosphide. The production of a sodium halide salt would be indicative that the metathesis reaction occurred.
As a preliminary examination of how the metathesis reaction would proceed, Na3BP2 was reacted with an equal molar ratio of a first-row transition metal chloride (M = V-Zn) in a sealed silica ampoule for 1 hr. If no reaction was observed at 500°C, the temperature was increased up to 700°C. In all cases, NaCl was produced. Commonly, metal phosphides and at least one unidentified phase were detected as products via powder XRD:
| T |
| H2O |
|
Na3BP2 + MCl3 | => | 3 NaCl + “NaMBP2” | => | MP + BP |
Washing the products with water to remove the halide salt appeared to destroy the new phase and cause binary BP to be newly detected by powder XRD. This suggests that the “NaMBP2” compound contained B-P bonds and that the compound decomposed into BP upon exposure to water or air. Control experiments discounted the possibility that BP came from degradation of Na3BP2. Further experimentation focused on the reaction of Na3BP2 with CuCl due to the high yield of new phase and the absence of binary byproducts.
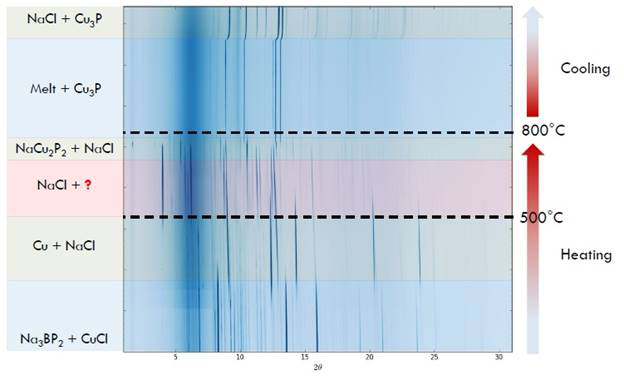
In-situ synchrotron XRD using the 17-BM beamline at the Advanced Photon Source at Argonne National Lab was used to analyze the reaction mechanism to gain insight on optimal reaction conditions (Fig. 1). Several beamlines were attended during the span of the project to optimize the experimental setup to our needs and to correct the obtained temperatures to be able to reproduce those conditions in the laboratory settings. Upon heating a mixture of CuCl and Na3BP2 in evacuated and flame-sealed silica capillary, Cu3P and Cu first formed as intermediates starting at 200°C along with the appearance of NaCl. The formation of new compound “NaMBP2” was observed around 430°C and both intermediates disappeared at 500°C. Doubling the heating rate seemed to prevent the formation of Cu3P, while reducing the heating rate revealed NaCu2P2 as another intermediate phase at 700°C. Neither the new compound nor NaCu2P2 crystallized upon cooling the melt with Cu3P crystallized instead. The temperatures obtained from these in-situ studies were corrected using several standards to account for the displacement of the sample and thermocouple in synchrotron setup. Though peak shift in powder XRD due to thermal effects make unit cell determination challenging at high temperatures, we hypothesized that new metal borophosphide “NaCu2P2B” has crystal structure similar to that of NaCu2P2 with additional B atoms linking P atoms from different layers. Similar results were obtained using MnCl2 as the transition metal halide with the formation of a “Na2Mn2P2B” intermediate. As with the Cu system, neither the new compound nor NaMnP recrystallized if the reaction was brought to a melt.
Attempts to synthesize the unknown compound using alternative routes, such as BI3- assisted reactions developed in the first part of the project for BP production, resulted in different products. Using BI3, P, and either CuCl or Cu tended to form Cu1.98P3I2 and CuI. This suggests that the unknown compound includes Na since that is the one element missing from the BI3 route. This also supports the idea that the compound is a B-stuffed modification of NaCu2P2. Based on this idea, we will be focusing more on systems that produces sodium metal phosphides to investigate whether boron is truly in the structure or not. If our hypothesis is correct, it opens the possibility of using Rietveld refinements on high-resolution powder XRD measurements using NaCu2P2 or similar compounds as starting structural models instead of solely relying on single crystal experiments to determine structure and composition. The obtained structural models will be confirmed using 10,11B and 31P solid-state NMR.
Figure 1. In-situ powder XRD of Na3BP2 reacting with CuCl.
Personnel impact:
During this project students involved were trained in advanced synthesis science including the application of the in-situ techniques to optimize the synthetic conditions.