Reports: ND755008-ND7: Sequence Controlled Polymers Using Solid Supported Chemistry
Christopher K. Ober, PhD, Cornell University
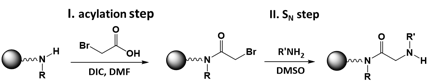
Sequence defined polymers were synthesized by solid supported peptoid chemistry following Zuckermann's submonomer synthesis. This reaction (Scheme 1) does not require any protecting group and allows an automated synthesis resulting in a good sequence control due to a simple and efficient purification by washing. New customized synthesis protocols and the use of a water jacketed reaction vessels enabled us to perform all experiments with our peptide synthesizer.
Scheme 1 Reaction scheme of Zuckmermann's synthesis to form peptoids.
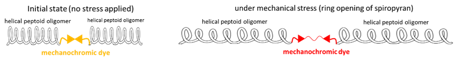
The structure of peptoid polymers can be controlled by choosing suitable side chains. Starting with a helix, investigation of sequence variation on helical pitch and the response to mechanical stress via helix extension is possible (Figure 1).
Figure 1 Possible color change of mechanochromic peptoid helices.
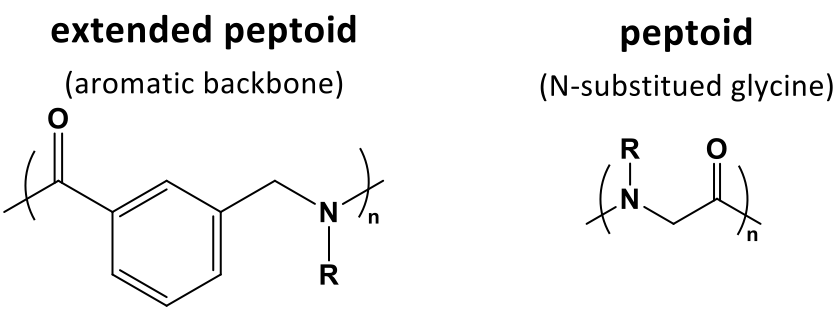
Our first intention was to synthesize extended peptoids with an aromatic unit in the backbone (Figure 2). These peptoids have a higher chain stiffness and a more uniform structure compared to normal peptoids (N-substituted glycines).
Figure 2 Chemical structure of extended peptoids and N-substituted glycine peptoids.
More than 50 % a-chiral amines are required to obtain a helix structure. Amines with aromatic units in b or g position were used as strong cis amide bond inducers to ensure a high homogeneity of the resulting peptoids.
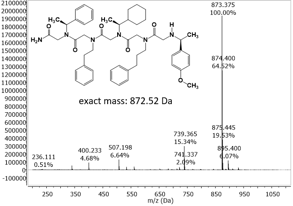
The use of the reactive Rink amide resin, long reaction times, high excess of reactants and high reaction temperatures in each reaction step resulted in an increase of the crude product yield in higher purity. Replacement of (S)-(-)-a-4-dimethylbenzylamine by the more acid stable (S)-(+)-1-cyclohexylethylamine successfully resulted in the first 5 letter peptoid sequence with an acceptable purity grade of 70 % (Figure 3).
Figure 3 Mass spectrum (ESI-MS) of a peptoid with 5 letter sequence.
Preliminary experiments to synthesize a chiral peptoid oligomer with three repeats of the 5 letter sequence were performed in collaboration with the Segalman group at the University of California, Santa Barbara. We discovered that the overall purity dramatically increases if the acylation step is performed twice, probably due to the large steric hindrance of the a-chiral amines and the aromatic substituents. However, a further modification of the sequence was necessary since the (4-methoxyphenyl)ethyl side chains in the middle of the backbone popped off during cleavage even if very low amounts of TFA (5%) were used. Reducing the sequence to 4 letters resulted in the first successful synthesis of a chiral peptoid oligomer (Figure 4 a).
a) |
b) |
|
|
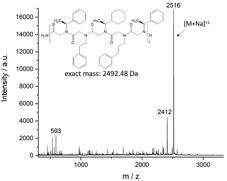
Figure 4 a) MALDI-TOF spectrum of 4 sequence peptoid oligomer (PO160520-22). Peak at 2412 Da resulted from a peptoid oligomer with only two (S)-(-)-a-methylbenzyl side chains, i.e. one of these side chains popped-off during measurement. b) CD Spectrum of the same peptoid oligomer at different concentrations in acetonitrile.
Adding (S)-(-)-1-(1-naphthyl)ethylamine to the sequence resulted in the first successful synthesis of a chiral 5 letter peptoid oligomer having an alternating chiral/nonchiral sequence (Figure 5 a). A second peptoid oligomer with the same monomers but with a chiral-block-nonchiral sequence was also successfully synthesized (Figure 5 b). This sequence variation might have a big impact on the helical pitch and should allow to analyze the influence of the sequence order on the mechanical properties of the peptoid oligomer.
a) |
b) |
|
|
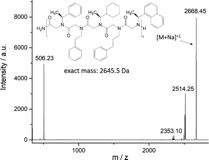
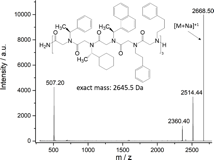
Figure 5 MALDI MALDI-TOF spectra of peptoid oligomers with 4 different letters. A) Sample PO160525-23; peak at 2514.25 Da = peptoid fragment with one popped-off (1-naphthyl)ethyl side chain, peak at 2353.10 Da = peptoid fragment having no (S)-(-)-a-methylbenzyl side chains. B) Sample PO160809-34; peak at 2514.44 Da = peptoid with one popped-off (1-naphthyl)ethyl side chain, peak at 2360.40 Da = peptoid fragment with two popped-off (1-naphthyl)ethyl side chains.
a) |
b) |
|
|
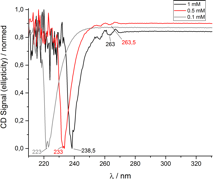
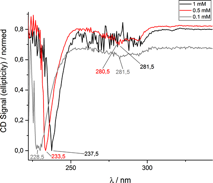
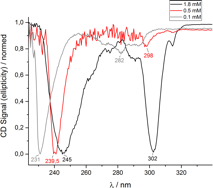
Figure 6 CD spectra of peptoids at different acetonitrile concentrations. A) Sample PO160525-23 with an alternating chiral/nonchiral sequence. B) Sample PO160809-34 with a chiral-block-nonchiral sequence.
The analysis of all peptoid oligomers via CD spectroscopy indicated helix formation (Figure 4 b, 6 a and b). The two CD absorbance maxima of the peptoid oligomers with the 5 letter sequence are typical for a right handed a-helix formation (Figure 6 a and b). However, with increasing concentration a bathochromic shift in conjunction with a shape change of the absorbance band was detected in all CD spectra for the first time for peptoids.
By comparing Figure 6 a and b it is clearly visible that the formation of the a-helix is dependent on the sequence order. The chiral-block-nonchiral peptoid oligomer in Figure 6 b showed a more distinct a-helix signal than in Figure 6 a. This probably indicates a slightly different structure (different pitch) and may result in different mechanical properties. Further studies will follow.
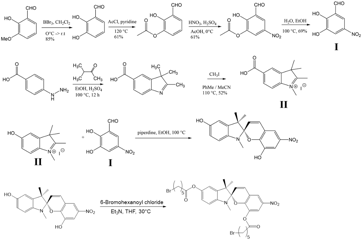
We also finished the multiple-step synthesis of a mechanochromic dye (Scheme 2 and Figure 7 a and b). The bromine functionalized spiropyran showed good solubility in THF, DMF and DCM, which is required for the following coupling with peptoid oligomers. It is our plan that the novel bifunctional mechanochromic dye will be used as a chemical linker between two peptoid oligomers to visualize the mechanical response under stress.
Scheme 2 Synthesis scheme of the bromine functionalized spiropyran.
a) |
b) |
|
|
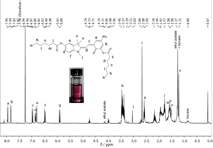
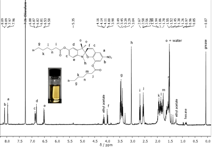
Figure 7 1H-NMR spectra of the mechanochromic dye in CDCl3. A) Open form. B) Closed form. UV irradiation results in the closed form while stress implies a ring opening reaction.
In summary, helical peptoid oligomers with 4 and 5 repeat sequences were successfully synthesized. End group functionalization of these materials with thiol or triple bonds was possible. The use of 5 different amines in the peptoid synthesis will offer 120 different combinations and may enable a precise control of the helical pitch and thus the mechanical properties.