Reports: UNI656095-UNI6: Electrochemical Study of Electronic Properties of Organic Thin Films in Orthogonal Electrolytes
Alex Zakhidov, PhD, Texas State University
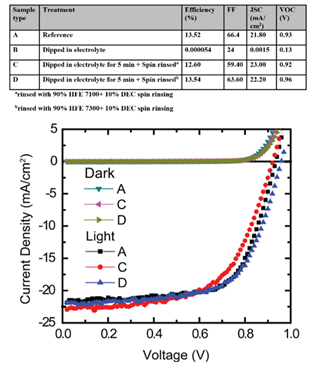
Fig. 1. J-V characteristics of solar cells with different electrolyte treatment as described in the Table above. The dark current density graphs are indistinguishable for all the types of sample. Negligible deviation in J-V graphs are evident between reference sample A and electrolyte treated sample C, D.
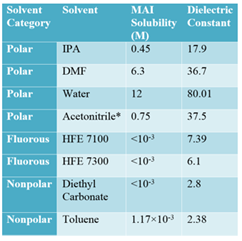
Table 1. List of maximum solubility of MAI in different polar, non-polar and fluorous solvents along with dielectric constant. *Measured in anhydrous acetonitrile in a nitrogen atmosphere.
Over the last few years, organic trihalide lead perovskites have been materials of extensive interest because of their rapid progress in optoelectronic devices. In addition to outstanding optoelectronic properties, extreme interest in perovskites are attributed to ease of processing and low materials cost. (M.A. Green, et al. Nat. Photonics 8, 506 (2014).) The simplicity of solution-based processing of perovskite materials have resulted in quick adoption in solar cells, thin film transistors (Y. Mei, et al, MRS Commun. 5, 297 (2015)), light emitting diodes (H. Cho, et al., Science 350, 1222 (2015)), light emitting field effect transistors (X.Y. Chin, et al, Nature Comm. 6, (2015)), lasers (H. Zhu, et al., Nat. Mater. 14, 636 (2015)), and more. While the properties of intrinsic perovskite materials are impressive, the delicate nature of these materials limits their ability to be precisely patterned or modified to tailor their structure and optoelectronic properties for specific applications. Furthermore, solution-based measurements of these films by electrochemistry can reveal fundamental aspects of these materials in solid state films, such as emergent energy levels and charge transfer rates. However, to date, such solution-based processing and measurements of solid-state perovskite materials have been limited as these materials are susceptible to dissolution in polar solvents, and nonpolar organic solvents do not support ionic transport for electrochemistry and ionic modification of films. Alternatively, an ideal fluid for processing and interrogation should not dissolve the perovskite. Furthermore, as an electrolyte, it should possess a high dielectric constant to dissolve a sufficient amount of ionic salt and support electrochemical measurements. In this manner, it should function as an “orthogonal solvent,” exhibiting solution behavior distinct from conventional polar and nonpolar solvents. Unfortunately, most solvents that have high dielectric constants dissolve either the perovskite material or its components. In addition to these properties, hydrogen-bond acidity, basicity, and dispersion forces should be considered, among other parameters. (C.M. Hansen, Hansen Solubility Parameters: A User’s Handbook, 2nd Ed. CRC Press, New York, 2007)
Fortunately, it has been demonstrated that chemically orthogonal processing of non-fluorinated organic films, both polar and non-polar, can be accomplished using highly fluorinated solvents such as hydrofluoroethers (HFEs). (A. Zakhidov, et al, Adv. Mater. 20, 3481 (2008)) Previous HFE treatments of various organic films did not cause any dissolution, cracking, delamination, or other unfavorable short term or long term physical or chemical damage. This was true even under extreme conditions, such as prolonged solvent exposure or elevated temperature processing. (A.A. Zakhidov, et al SID Symp. Dig. Tech. Pap. 42, 1740 (2011))
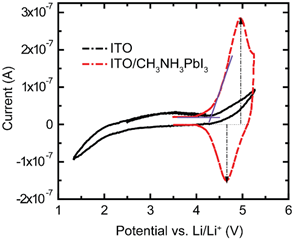
Fig. 2. Cyclic voltammetry of ITO/CH3NH3PbI3 and bare ITO in LiTFSI electrolyte. ITO shows no peak for oxidation and reduction. For ITO/CH3NH3PbI3 oxidation peaks are observed.
Moreover, one can use HFE solvents in concert with a small addition of co-solvents to make functional electrolytes, as demonstrated with lithium based salts having fluorinated organic anions.(G. Nagasubramanian et al, J. Power Sources 196, 8604 (2011)) HFE based electrolytes are promising for improving lithium ion battery safety and performance due to their nonflammability, high voltage stability window, less gas decomposition, modest viscosity, low freezing temperature, high vapor pressure, and low surface tension. In addition, HFEs are green fluorinated solvents with low toxicity that do not disturb the ozone. As such, these solvents are thus candidates for replacement of chlorofluorocarbons (CFCs) hydrochlorofluorocarbons (HCFCs) and perfluorocarbons (PFCs).
In this report, we demonstrate the selection and optimization of a solvent based on an HFE for electrochemical characterization of a perovskite films, particularly methylammonium lead iodide benchmark perovskite (CH3NH3PbI3 or MAPI). A series of solvents (Table 1) were investigated to balance low solubility (of the most sensitive organic component CH3NH3I or MAI) with high dielectric behavior, revealing the superiority of an HFE approach. Subsequently, the solubility and conductivity of lithium bis (trifluoromethane) sulfonimide (LiTFSI) salt in solutions of varying HFE 7100 and diethyl carbonate (DEC) ratios were determined, and electrochemistry performed with an optimal mixture. Cyclic voltammetry (CV) measurements revealed quasi-reversible oxidation of MAPI films in this electrolyte, and the highest occupied molecular orbital (HOMO) level was calculated from the oxidation onset (Fig. 1). Finally, the stability of the perovskite materials in the HFE electrolyte was further confirmed by comparing the performance of solar cells with and without electrolyte treatment on MAPI perovskite films (Fig. 2). It was found that full operation could be restored following HFE exposure by a rinsing procedure to remove residual lithium ions. The minimal change of efficiency, open circuit voltage, and short circuit current with HFE electrolyte treatment suggests that HFE can be used for electrochemical processing and characterization of perovskite films.