Reports: DNI955159-DNI9: Systems Level Design of Osmotically Driven Membrane Processes for Process Water Reuse
William A. Phillip, PhD, University of Notre Dame
As the production of nonconventional petroleum resources grows, so does the water demand of these processes. This necessitates the development of a technology that can handle complex industrial water streams in order to enable the reclamation and reuse of produced and process waters. Forward osmosis (FO) is a subset of osmotically driven membrane processes that has potential applications in this critical arena. Therefore, in the first year of the project, we derived a predictive model to inform the design of a FO module as a function of operating conditions and membrane properties. Experiments corroborated the predictive ability of the model, as long as the system functioned under the assumed constraints of non-interacting solutes. In the second year of our efforts, membranes with chemistries designed to engender solute-membrane interactions were developed to experimentally investigate the potential impact of these multicomponent interactions. During these experimental efforts, we noticed that it may be possible to design these membranes such that they could separate molecules based on chemical factors, rather than physical factors. Because this is one promising approach to meet the demand for highly-permeable membranes that are more selective, progress in this area of our effort is discussed below.
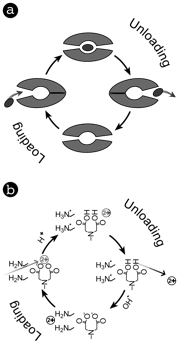
The design of multifunctional, pH-responsive membranes that selectively pump a target solute is described. The membranes consist of a gate layer made from an amine-functionalized copolymer and a reactive matrix lined by iminodiacetic acid groups, which bind divalent cations reversibly (Figure 1). These chemistries exhibit concurrent changes in the cation binding affinity and gate permeability in response to the pH value of the surrounding solution such that when they are exposed to an oscillating pH, the combination drives a facilitated transport mechanism that mimics ion pumps found in natural systems (Figure 2).
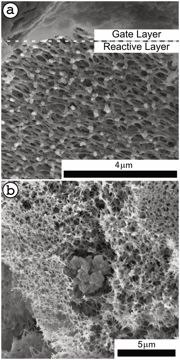
Figure 1. Cross-sectional scanning electron microscopy (SEM) micrograph of the composite polymeric ion pump. a) A dense copolymer comprises the gate layer, which sits on top of the reactive layer containing chelating resin dispersed in phase inverted polyacrylonitrile (PAN). b) The porous polymeric material of the reactive matrix surrounds a grain of ground resin lined by iminodiacetic acid ligands.
Figure 2. Schematic of the ion pumping mechanism driving transport. The mechanism relies on a cyclic process that consists of loading and unloading stages where the conformation of the membrane directs the flux of solute in a prescribed direction. During the loading cycle, the feed side of the membrane, which is coated with neutral amine functional groups, allows solute to flow into the membrane where it is captured by iminodiacetic acid groups that are dispersed throughout the matrix of the membrane. Subsequently, an external stimulus causes the amine groups to protonate and form positively-charged ammonium groups, which makes the feed side of the membrane impermeable to cations. Concurrently, the stimulus causes the solute trapped within the membrane to be released and directed toward the receiving solution. In this study, solution pH was the stimulus used to periodically cycle between the two states.
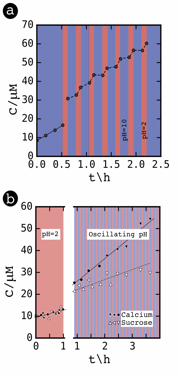
In mixed solute systems, calcium permeated through the membrane four times faster than sucrose in the presence of an oscillating pH even though the solutes possess similar hydrodynamic sizes and permeated through the membrane at the same rate when the pH value was constant (Figure 3).
Figure 3. The effect of oscillating pH on solute permeation. a) The concentration of calcium permeating into the receiving solution is plotted as a function of time. The black dots indicate measured values of calcium concentration. The background color specifies the pH value of the feed solution. A blue background represents a pH value of 10; a red background represents a pH value of 2. The oscillations were initiated at t = 0.5 h. b) The concentrations of calcium and sucrose in the receiving solution are plotted as a function of time. The feed solution contained both solutes at a concentration of 10 mM. For the first hour, the pH value of the feed solution was held constant. Subsequently, the pH value of the feed solution oscillated between pH 2 and pH 10. A loading time of 525 s and an unloading time of 281 s were used.
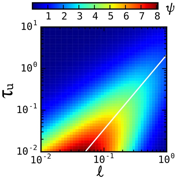
The development of the polymeric ion pumps was guided by a model that provided several critical insights. First, the solute binding capacity (i.e., the number of iminodiacetic acid moieties dispersed throughout the reactive matrix) and thickness of the membrane define the asymptotic limit for enhanced selectivity. Second, the maximum enhancement in selectivity is realized in the limit of infinitely rapid oscillations (Figures 4 and 5).
Figure 4.
The performance, ψ, for a membrane with a dimensionless capacity of 10 is plotted as a
color gradient for the phase space of unloading times ![]() and reactive front locations
and reactive front locations ![]() . The reactive front location is directly related to the length of the
loading period. The performance of the membrane is defined as the solute flux
realized in the oscillating system normalized by the solute flux in a purely
diffusive system. The pair of
. The reactive front location is directly related to the length of the
loading period. The performance of the membrane is defined as the solute flux
realized in the oscillating system normalized by the solute flux in a purely
diffusive system. The pair of ![]() and l
and l![]() that maximize performance is
identified by the diagonal white line.
that maximize performance is
identified by the diagonal white line.
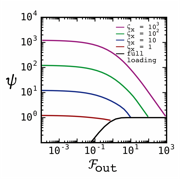
Figure 5. The
performance of an optimized system, the white line, is plotted as a function of
its normalized flow of mass. Membrane capacities ranging from 1 to 1000 were
examined. In all cases the plot is bounded asymptotically by the limit of ![]() on the left and by the
full-loading limit on the right.
on the left and by the
full-loading limit on the right.
The multifunctional membranes discussed here provide a platform for the development of processes that can fractionate molecules of similar size but varying chemistry. Moreover, similar membranes are allowing us to investigate the effects of multicomponent interactions on the design of forward osmosis modules.
This research project has made a significant impact on the development of graduate and undergraduate researchers at the University of Notre Dame. It has help expand the participation of undergraduate student researchers so that future scientists may connect theoretical principles with hands-on experimentation.