Reports: ND456375-ND4: Probing Interactions between Hydrocarbons and Auxiliary Guests inside Cucurbit[8]uril Using Fluorinated Metal-terpyridine Complexes
Eric Masson, PhD, Ohio University
1. Introduction
The long-term goal of this project is to develop assays to characterize complex hydrocarbon mixtures such as crude oil batches. To that aim, we designed a supramolecular complex formed upon interaction of the macrocycle Cucurbit[8]uril (CB[8]) and of a fluorinated probe bearing a CB[8]-binding unit. The cavity of CB[8] is large enough to accommodate both the probe and a hydrocarbon analyte. The nature of the CB[8]-binding unit thus impacts the selectivity of the probe towards the analytes. Hydrocarbon binding is monitored by nuclear magnetic resonance (NMR), UV-Vis and fluorescence spectroscopy.
2. Impact
So far, this grant has allowed a second year graduate student to publish his first article (Org. Lett. 2017, 19, 4303). The same graduate student, as well as two Ohio University undergraduate students and an exchange student from the University of Mulhouse, France, have gathered enough data to generate two more publications in the coming months. This ACS PRF grant is having a tremendous impact on our research output, and is paving the way for the preparation of a US Department of Energy (DOE) grant.
3. State of Research
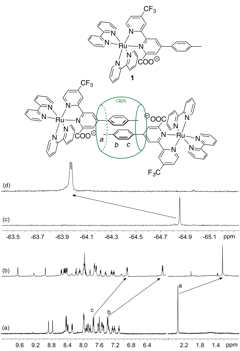
The first hydrocarbon probe we designed was Ruthenium(II) tris(2,2′-bipyridyl) complex 1, with one of the bipyridyl ligands bearing (1) a tolyl group at position 4′ as CB[8]-binding unit, (2) a carboxylate substituent at position 6′ to weaken CB[8] binding, and (3) a trifluoromethyl group at position 4, to allow the monitoring of the binding mechanism by 19F NMR spectroscopy. Guest 1 formed a homo-ternary complex in the presence of 0.50 equiv CB[8] in deuterium oxide, as shown by both 1H and 19F NMR spectroscopy (see Figure 1).
Figure 1. Formation of homo-ternary complex 12.CB[8]. (a) 1H NMR spectrum of Ru complex 1; (b) 1H NMR spectrum of complex 12.CB[8]; (c) 19F NMR spectrum of Ru complex 1; (d) 19F NMR spectrum of complex 12.CB[8].
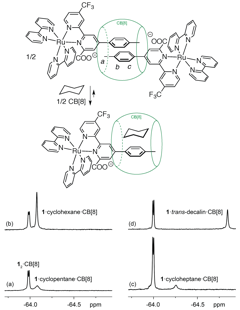
A series of 20 liquid hydrocarbons were then added (in excess) to the homo-ternary complex, resulting in their encapsulation by the CB[8]/auxiliary guest pair and the partial formation of hetero-ternary complex 1.H.CB[8]. The binding process is clearly monitored by 19F NMR spectroscopy, with the appearance of a new signal pertaining to hetero-ternary complex 1.H.CB[8] (see Figure 2). Remarkable selectivity was also observed: all tested cyclic hydrocarbons except cyclodecane underwent encapsulation (C5-C8 as cycloalkanes and cycloalkenes), while aliphatic members of the series (C5-C10) did not. The probe responds to guest volume, as cyclohexane binds better than cyclopentane and cycloheptane, and cycloheptene better than cyclopentene and cyclohexene. Cis- and trans-decalin interacted very strongly, up to 8,700 times better than benzene, and at least 105 times better than parent cyclodecane. More surprising is the selectivity of the probe for cycloalkanes, compared to cycloalkenes and benzene; in fact, the latter displays the weakest affinity for the probe among the series of interacting hydrocarbons.
Figure 2. Equilibrium between homo-ternary complex 12.CB[8], hydrocarbons, CB[8] and hetero-ternary complex 1.H.CB[8]. Monitoring of the recognition process by 19F NMR spectroscopy for (a) cyclopentane, (b) cyclohexane, (c) cycloheptane and (d) trans-decalin.
We then assessed in detail the thermodynamics of the recognition process, and showed that both the desolvation of the hydrocarbon upon encapsulation and the dispersive interactions between the hydrocarbon, the auxiliary guest and the inner wall of CB[8] regulate the binding affinity. To isolate both terms, we showed that instead of considering the equilibrium between the CB[8]/auxiliary guest pair and the hydrocarbon in aqueous solution, we could assess the equilibrium between the pair in solution and the hydrocarbon in the gas phase, thereby removing the impact of hydrocarbon desolvation on the interaction. As far as interactions between auxiliary guest 1, CB[8] and the hydrocarbon are concerned, we could then show that CH-pi interactions between the aromatic (tolyl) unit of auxiliary guest 1 and the hydrocarbons contribute significantly to the binding affinity, while pi-pi interactions (with benzene, for example) are inexistent. The last statement nicely supports recent computational results indicating that interactions between units bearing less than 10 atoms each are dictated by the geometry of the assembly, and not by interactions between pi orbitals.
4. On-going and future studies
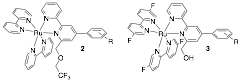
As mentioned in our Organic Letters article, the synthesis of guest 1 is time consuming, and the purification of several intermediates leading to its preparation is particularly tedious. We are thus refining the structure of our probes to allow rapid synthesis and diversification of the CB[8]-binding unit. Guests 2 and 3 are currently being prepared, and their binding towards hydrocarbons (including terpenes present in natural oils) are being assessed. Progress will be reported in due course.