Reports: ND356126-ND3: Secondary Coordination Sphere meets Secondary Bonding: Novel Ligand Design and Catalysis
John Protasiewicz, Case Western Reserve University
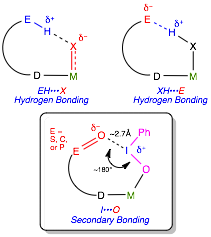
Introduction This work concerns the careful design and synthesis of ligands and metal complexes that possess appropriately located functionalities with strong dipoles (C=O, P=O, and S=O units) to create a secondary coordination sphere that can engage in secondary bonding with substrates (Figure 1, bottom). Previously reported ligands with secondary coordination sphere functionalities have used hydrogen bonding as a directing tool (Figure 1, upper).
Figure 1.
Our work targets hypervalent iodine reagents such as iodosylbenzene that are insoluble in common organic media due to their solid state aggregation by secondary bonding (I···O interactions). The outcomes and impacts of the proposed research include the discovery of the fundamental design principles for introducing secondary bonding into the secondary coordination sphere that could lead to advances in the rational synthesis of new catalysts that utilize hypervalent main group species. There are three specific main aims for this proposal.
i. Prepare ligands for potential use for secondary bonding in the secondary coordination sphere
ii. Synthesize and characterize new complexes in secondary bonding in the secondary coordination sphere
iii. Bind hypervalent iodine reagents to complexes
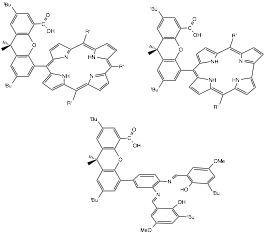
Year 1 Progress We have made progress on the first two aims above. Our initial work has concentrated on Nocera-type "Hangman" porphyrin complexes that ligands that dangle acid-base functional groups in the secondary coordination sphere for hydrogen bonding (Scheme 1).
Scheme 1. Three selected examples of hangman ligands.
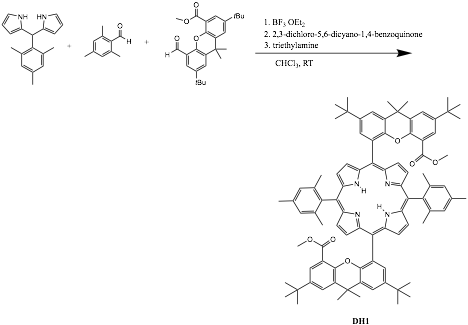
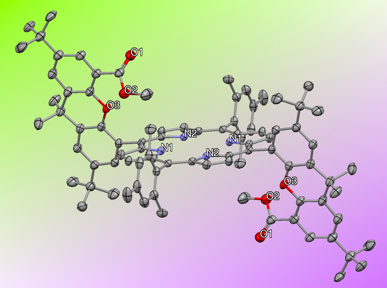
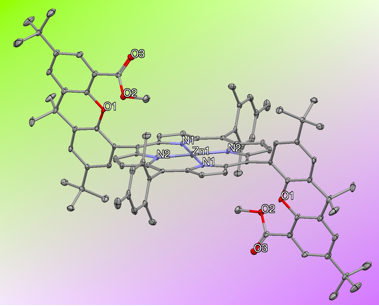
During our efforts to follow the protocols developed by the Nocera group for synthesis of these types of porphyrins (Scheme 2), we found that "double" hangman porphyrin ligand DH1 (Figure 2) and a zinc complex (Figure 3) can be made and structurally characterized. The doubly functionalized porphyrin is expected to be even more suited to our purposes to make stable metal-iodosylbenzene complexes.
Scheme 2.
Figure 2.
Figure 3.
With these materials in hand, we are now beginning to explore binding of hypervalent iodine reagents, such as iodosylbenzene, to these complexes. This route is general and will allow is to vary the meso-substituents from mesityl to the penta-fluorophenyl, phenyl, and p-anisole derivatives to find the right combination of factors that will allow isolation fo the desired final compounds that can be crystallized. We plan to also prepare analogous iron, cobalt, and nickel complexes and examine them by single crystal X-ray diffraction methods. The complexes will then be reacted with one equivalent of iodosylbenzene and their reactivity explored.