Reports: DNI755456-DNI7: Miscibility and Structures of Polycyclic Aromatic Hydrocarbons and Polymeric Materials
Sangwoo Lee, Rensselaer Polytechnic Institute
We investigated the miscibility of model polyaromatic hydrocarbon (PAH) compound and polymers with aromatic side groups and without aromatic side groups. Despite PAHs are abundant and frequently generated in many systems ranging from combustions to crude oils, systematic investigation of the thermodynamic miscibility with another class of compounds is rare. PAHs have unique electronic structures, which makes this class of compound thermally stable and display useful optical properties. However, PAHs have planar molecular structures, and the strong π-π interactions make these compounds highly immiscible with other compounds.
We investigated the miscibility of pyrene as a model PAH compound and polymers with and without aromatic side groups. Figure 1 shows the molecular structures of pyrene and the polymers employed in this study. For the systematic investigation of miscibility between pyrene and these polymers, we extracted the effective interaction parameters between pyrene and the model polymers. Quantifying interaction parameters is very important to understand the phase and microscopic behaviors of polymer mixtures because the interaction parameters state the macroscopic phase states of mixtures and microscopic states of polymer chains. By combining the Flory-Huggins theory and equality of chemical potentials of crystallizable compounds at crystal melting points in binary mixtures, we extracted the interaction parameters using the following relationship:
where Tm and Tm,PY are the melting temperatures of pyrene crystals in mixtures and pure pyrene, respectively; R is the gas constant; ΔHm,PY is the melting enthalpy of pyrene crystal; ϕp is the volume fractions of the polymer; N is the number of lattice sites occupied by a polymer chain, which is the degree of polymerization based on the volume of a unit pyrene molecule; and
is the effective interaction parameter where A0, A1, and B are parameters. The first two terms of in the right hand of the χ represent the non-combinatorial entropy contribution in the mixing, which is not taken account into the combinatorial entropy part in the Flory-Huggins theory. The last term of the Eq. 2 represents the enthalpic contribution in the mixing. The Eq. 1 enables easy extractions of the effective interaction parameters between crystallizing compound and non-crystalline polymer.
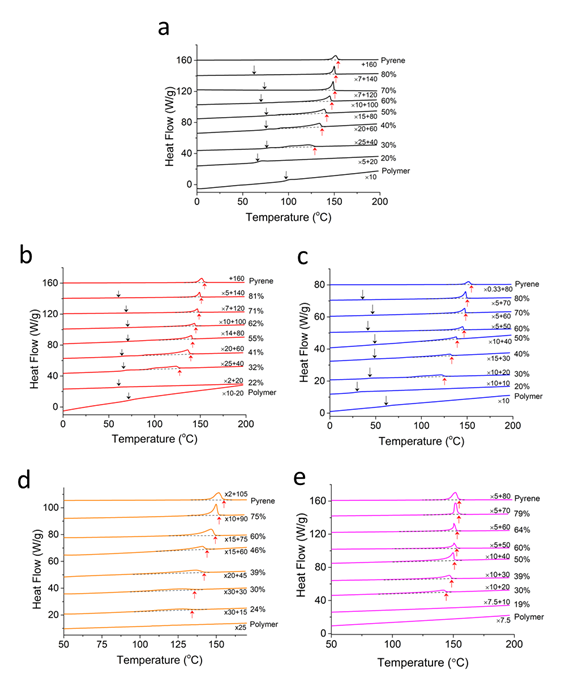
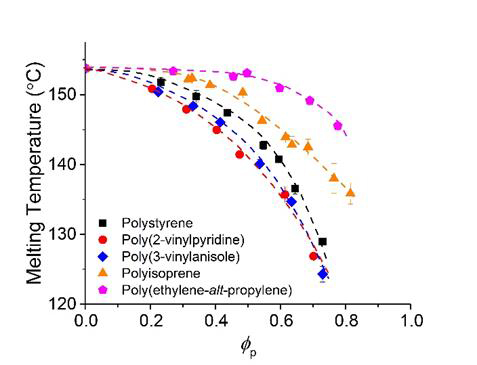
We extracted the effective interaction parameters between pyrene and model polymers using the differential scanning calorimetry (DSC) characterizations. Figure 2 shows the DSC thermograms obtained from final heating steps of pyrene and polymer mixtures. The mixtures were annealed at 85 °C for 5 hours and cooled down to -50 °C before the final heating. Using the melting points of pyrene crystals marked by up-arrows in Figure 2, we constructed the phase portraits of extracted the effective interaction parameters. Figure 3 shows the phase portraits of the binary mixtures of pyrene and the model polymers. The differnet melting points at a same composition indicate different compatibility between pyrene and these polymers. Lower melting point of pyrene crystals suggests higher compatibility with the polymer. The phase boundaries suggest that the compatibility with pyrene decreases in the order of poly(2-vinylpyrene), poly(3-vinylanisole), polystyrene, poly(1,4-isoprene), and poly(ethylene-alt-propylene) polymers.
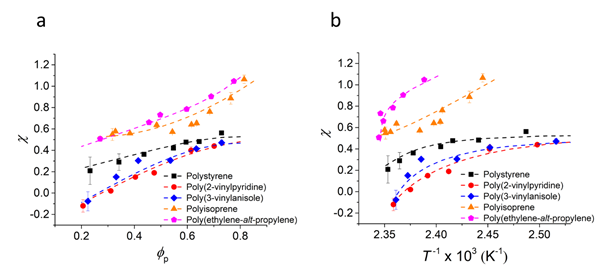
Indeed, the interaction parameters extracted using the melting points of pyrene crystals shown in Figure 4 also consistent with the order of phase boundaries. Interestingly, the interaction parameters appear linear functions of compositions, which suggests that certain microscopic interactions between pyrene and polymer segments contribute to the interaction parameters. Interestingly, the slope of linear dependence of χ on ϕp becomes smaller for the χ close to 0.5, which represents the athermal condition. This indicates that a copolymer of styrene and isoprene may show composition-independent interaction parameter with pyrene, χ = 0.5.
The most remarkable advantage in the use of thermal characterizations in the characterization of thermodynamic properties of a binary mixture containing a crystallizing component is that the extraction of the composition of non-crystalline phase is possible. In this study, the pyrene and polymer binary mixtures investigated were all annealed at 85 °C for 5 hours. The endothermic peaks in the DSC thermograms shown in Figure 2 start approximately at 85 °C and complete at the melting points of pyrene crystals. Thus, the endothermic peaks practically represent the heat associated with the melting and mixing of pyrene crystals at 85 °C. Thus, the energy of the endothermic peak can be correlated with the Gibb energy of mixing (ΔGmix) and the crystallinity of pyrene (fc) at 85 °C as follows:
Where MPY is the molecular weight of pyrene and wPY is the weight fraction of pyrene in the mixture.
Using
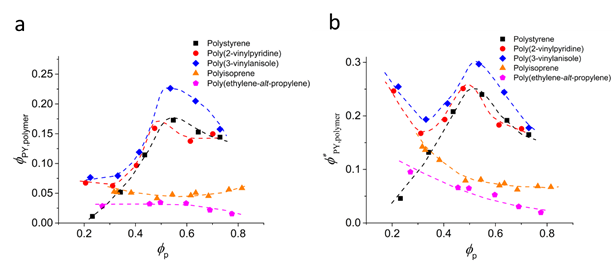
the Eq. 3, we extracted the composition of polymer phase at 85 °C. Figure 5
shows the volume fractions of pyrene dissolved in the polymer-rich phase
relative to the total volume of the mixture (
![]()
![]()
The understanding gained from this study will be helpful to design polymeric materials to control the phase states of PAH-containing materials such as asphaltene in crude oils.
Figure 1. Molecular structures of pyrene and the polymers employed in this study.
Figure 2. DSC thermograms of mixtures of pyrene and (a) polystyrene, (b) poly(2-vinylpyridine), (c) poly(3-vinylanisole), (d) polyisoprene and (e) poly(ethylene-alt-propylene.
Figure 3. Phase portraits of binary mixtures of pyrene and model polymers.
Figure 4. Effective interaction parameters (χ) between pyrene and model polymers.
Figure 5. Pyrene compositions in the two-phase states at 85 °C based on the heat of melting of the pyrene crystal.