Reports: DNI757095-DNI7: Study of New Polybetaine's Self-Assembly and Their Effect on Paraffin Inhibition
Nurxat Nuraje, Texas Tech University
Narrative Progress Report
Overview:
The proposed work focuses on synthesizing Polybetaines with different hydrocarbon groups and studying both their assembly in different solvents and their inhibition mechanism of paraffin crystallization. The specific objectives are (1) to synthesize Polybetaines with different alkyl lengths(HMPBs) and to characterize them using NMR, FTIR, and GPC; (2) to study their assembly properties in different conditions including different pHs, solvents with different polarity, ionic strengths, and temperatures using cryo-TEM, DLS, and viscometer; (3) to understand the effect of hydrophobically-modified polybetaines on the nucleation and crystal growth of paraffin through characterizations using microscopy techniques. The proposed work is anticipated to establish a new class of polybetainic paraffin inhibitors, a new understanding on the inhibition of paraffin crystallization and guidelines for designing paraffin inhibitors for crude oils.
Tasks of project (from 07/01/2016—08/31/2018):
Task 1 (2016- 2017): We will synthesize novel monomers and HMPBs (from С8 to С18). The composition and Molecular weights of synthesized monomers and polymers will be further confirmed by NMR, FTIR, and GPC. In parallel with the above random radical polymerization (RRP) synthesis, Reversible addition-fragmentation chain transfer (RAFT) will be developed to synthesize these HMPBs with controlled molecular weight. The NMR and GPC techniques will be used for characterization of these polymers and their polydispersity.
Task 2 (2016- 2017): Self-assembly of Polybetaines: Hydrodynamic, conformational, molecular, and thermal properties of monomers and HMPBs (C8-C18) will be investigated by DLS, zetasizer, cryo-TEM, GPC.
Task 3 (2017- 2018): The Paraffin inhibition mechanism of the HMPBs: The physical-chemical and rheological characteristics of model oil and oil mixtures in particular, the dynamic and kinematic viscosity, paraffin content, paraffin crystal structure, - will be studied.
For rheological studies, model waxy oil samples are prepared from paraffin waxes in decane, and straight chain alkanes of carbon numbers (Cn) n = 24, 28, 32, and 36 as paraffin waxes will be examined.
Pour points will be measured according to ASTM D97 and ASTM
Wax crystal morphologies will be determined with a polarized microscope.
Wax deposition inhibition: Inhibition of wax deposition was tested on a “Cold Finger” test.
Results of First Half- Year (from 07/01/ 2016- 08/31/2017):
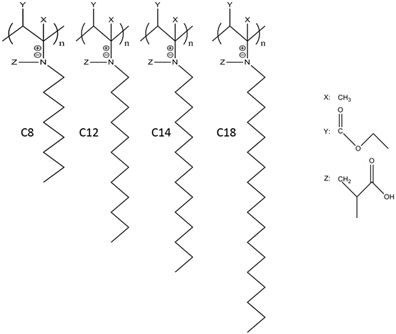
We have accomplished task 1 and task 2 according to our research plan. The HMPBs (C8- C18) (Figure 1) were successfully synthesized via both RRP and RAFT approaches.
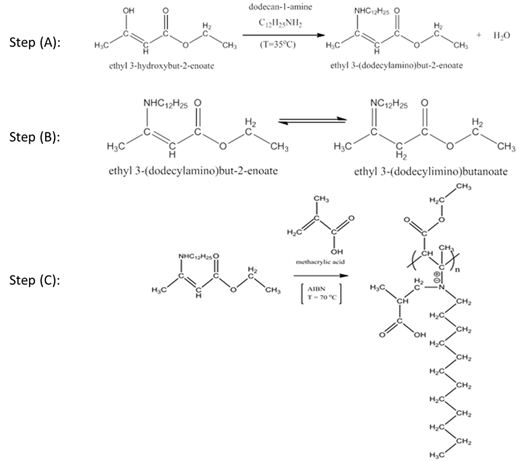
In the synthesis of HMPBs, as shown in Figure 2, the monomers were first synthesized before polymerization. Briefly, the typical monomer synthesis: premelted alkylamines were mixed with acetoacetic ester (AAE) under the stirring during 3h at 60°C. Resulting alkylaminocrotonates were produced with high yield (>95%). These monomers formation was also confirmed and FTIR and NMR.
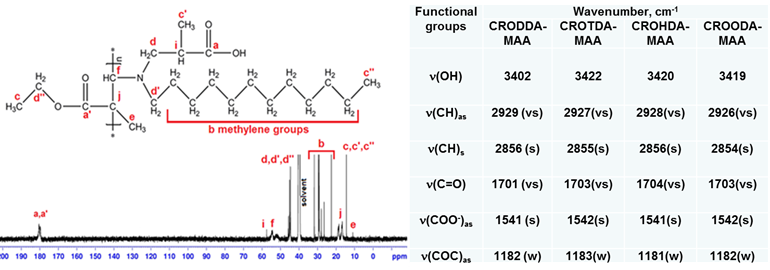
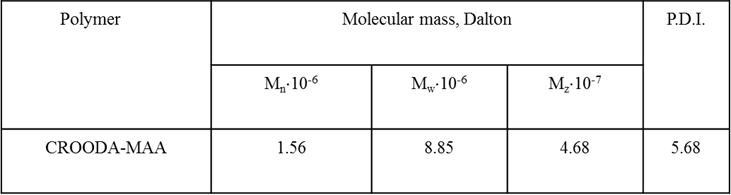
Polymerization of alkylaminocrotonates and methacrylic acid (MAA) was carried out in benzene at 70°C in the presence of azoisobutyronitrile (AIBN). Addition of MAA to alkylaminocrotonates leads to formation of betaine monomers that undergo radical polymerization. The radical polymerization of 3-[(2-carboxypropyl)alkylamino]-but-2-enoic acid ethyl ester leads to formation hydrophobically-modified linear polybetaines with hydrocarbon (C8, C12, C14, and C18) abbreviated as CROODA-MAA, CROTDA-MAA, CROHDA- MAA and CROODA-MAA, respectively. Both NMR and FTIR techniques were applied to confirm the structures of HMPBs. Figure 3 shows the NMR spectra of CROODA-MAA. The HMPBs synthesized via RRP approach have high molecular weight (1, 000,000 g/mole) and large polydispersity index (PDI: 5.6) (Table 1).
In the RAFT polymerization of the HMPBs, the only extra chain transfer agent (2-Cyano-2-propyl dodecyl trithiocarbonate, CTA) was added at certain ratio of CTA: AIBN. The rest of the experimental procedure was the same as RRP’s. Likewise, NMR and FTIR were applied to confirm the composition of the HMPBs. Both PDI and MWs were controlled to be 1.2 and 78000 g/mol respectively.
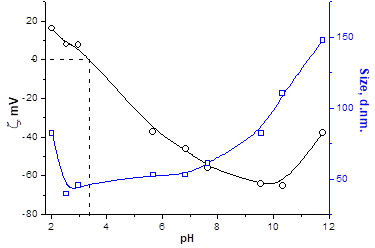
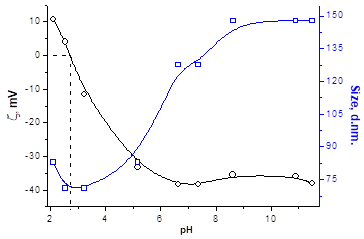
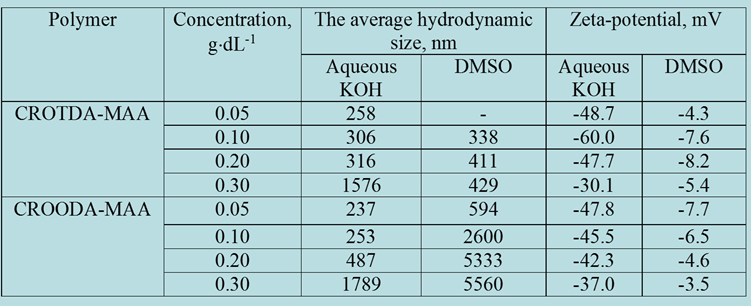
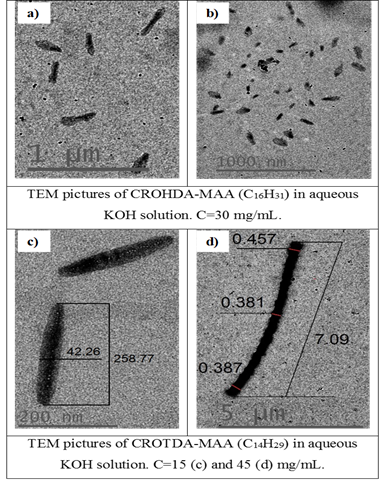
DLS, Zeta Potential, and TEM Study of HMPBs: By using DLS and Zetasizer instruments on the synthesized HMPBs, for example, CROTDA-MAA and CROODA-MAA, the average hydrodynamic size and zeta potential were determined as a function of pH. The average hydrodynamic size of CROTDA-MAA in aqueous solution ranges from 40-150 nm. According to zeta potential, the Isoelectric point (IEP) of a 0.1wt % aqueous solution of CROTDA-MAA is 3.4 (Figure 4).
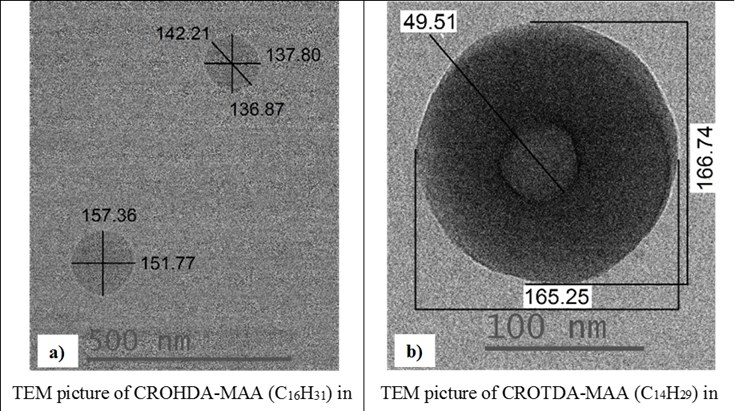
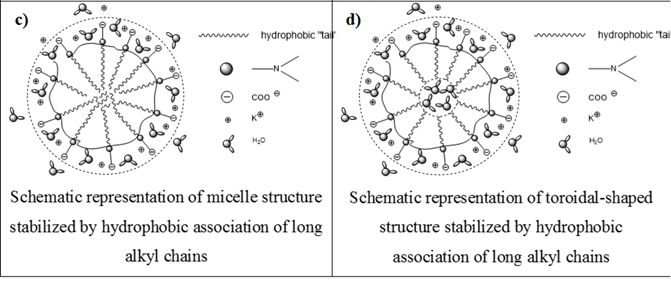
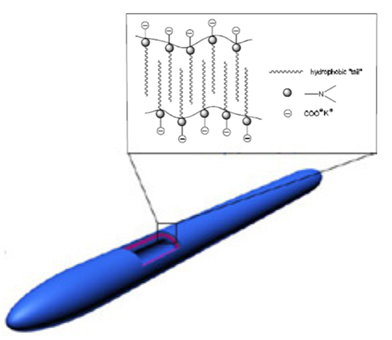
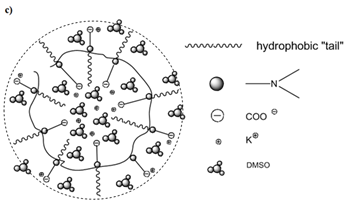
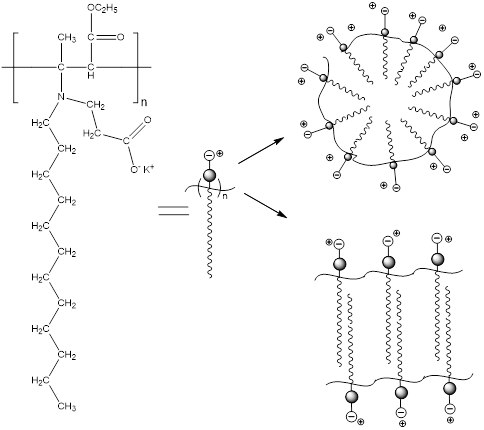
The IEP for CROODA-MAA is 2.7. The average hydrodynamic size is in the range of 70-150 nm depending on pH (Table 2). At the same time, the self-assembly of HMPBs observed by TEM was found to be spherical structures in DMSO. However, in the 0.1 KOH solution, the HMPBs had different self-assembly structures. At the low concentration HMPBs formed spherical micelle, but with increase of the concentration of the HMPBs, the self-assembly structures were transferred from spherical to cylindrical micelle ( Figure 5, 6, and 7) . Figure 8 explained that their self-assembly structures in 0.1 N KOH.
In summary, according to our research plan, HMPBs were successfully synthesized via RRP and RAFT approaches. The chemical compositions of the HMPBs were confirmed by FTIR and NMR. Their MWs (and PDI) synthesized by both RRP and RAFT approaches were found to be around 1, 000,000 (g/mole) (and 5.6), and 78000 kg/mole (and 1.2). Self-assembly of the HMPS were studied by DLS, zeta potential and cryo-TEM. In the DMSO, HMPBs were mainly formed spherical micelle structures. But in the aqueous solution, they formed two different micelle structures with spherical and cylindrical with concentrations of HMPBs. Next, we plan to study the effect of HMPBs on paraffin inhibition mechanism as planned in Task 3.
Figure 1. HMPBs with C8, C12, C14, and C18 alkyl groups.
Figure 2. Polymerization of Polybetaines.
Figure 3. (Left) NMR 13C and (Right) FTIR spectra of HMPBs.
Figure 4: Size and zeta potential data for CROTDA-MAA (left) and CROODA-MAA (right).
Table 1: The molecular Weight
Table 2: The average hydrodynamic size and zeta-potential
Figure 5. TEMs of CROHDA-MAA (a) and CROTDA-MAA and the schematic illustration.
Figure 6: TEM pictures of CROHDA-MAA (a,b) and CROTDA-MAA (c,d) at 30 mg/mL (a,b), 30 mg/mL (c) and 45 mg/mL (d).
Figure 7. TEM of CROTDA in DMSO and schematic representation of micelle structure. C=7.5 mg/mL.
Figure 8. Scheme for micellar structures in aqueous solutions.