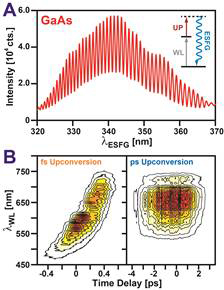
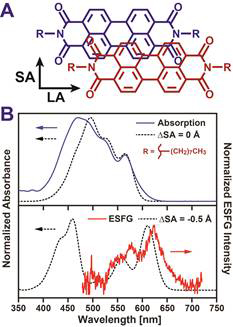
Reports: DNI655184-DNI6: Visualizing Molecular Organization and Energy Transport Dynamics at Organic Surfaces and Heterojunctions with Interface Specific Femtosecond Spectroscopy
Sean T. Roberts, PhD, University of Texas, Austin
Organic semiconductors (OSCs) are a unique class of petroleum-derived materials that blend the processing advantages of plastics with the electrical properties of semiconductors. In contrast to inorganic semiconductors such as silicon, OSCs can be readily processed from solution into highly-absorbing, thin, conductive films, making OSCs attractive for a wide range of optoelectronic applications. In many of these applications, the transfer of charge to and from OSCs is fundamental to device operation. Our goal is to develop interface selective spectroscopies that can investigate OSC interfaces and establish how the molecular organization of these regions controls their ability transfer charge. The suite of techniques we have worked to develop utilize electronic sum frequency generation (ESFG) to achieve interfacial selectivity. In an ESFG measurement, two pulses of light, a white light (WL) continuum and a narrowband upconversion pulse illuminate a sample, producing a new light pulse at their sum frequency (Figure 1A inset). Resonance between frequency components of the WL and electronic transitions within a sample strongly enhances this mixing, allowing ESFG to serve as a proxy for the sample’s electronic absorption spectrum. Importantly, due to the cancellation of forward- and back-propagating fields, ESFG is preferentially created by regions of a sample that lack inversion symmetry. As OSC interfaces naturally lack such symmetry, ESFG can selective examine the structure and dynamics of these critical regions. Over the last year, we have achieved two key milestones described below: