Reports: UR1053827-UR10: Bringing Undergraduates Into Lab-Based Research and Development (BUILD) for Effective Shale Gas Storage Using Macroporous Adsorption Complexes (MACs)
Jingbo Liu, PhD, Texas A&M University, Kingsville
Shale gas introduction: The shale gas as an important source of natural gas has been studied in many countries. Among these countries, the US and Canada have significant shale gas production although other countries have large amount of the recoverable shale resources. The shale is a fine-grained sedimentary rock that forms from the compaction of silt and clay-size mineral particles. One of the major components composed of the shale gas is methane (CH4). Suitable material designed for efficient methane storage is the key perspective in this study. To improve the CH4 storage, its main features as an energy source are needed to be fully understood, such as at room temperature, CH4 is supercritical. Another main characteristics of methane (Tc = 190.6 K, Pc = 45.8 atm) indicated that CH4 cannot be liquefied by compression above Tc. Herein, it is challenging to store the large amount of CH4 in a given volume for its portable application, such as gas-driven automobile. Preparation of porous materials therefore becomes important to achieve the above end application of CH4. This report summarized series materials prepare by a feasible wet-chemistry approach and the variable optimization. The gas uptake was also measured to evaluate their porosity and surface area.
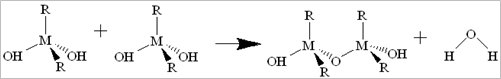
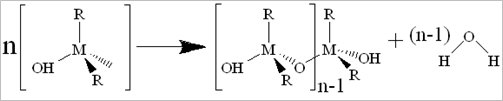
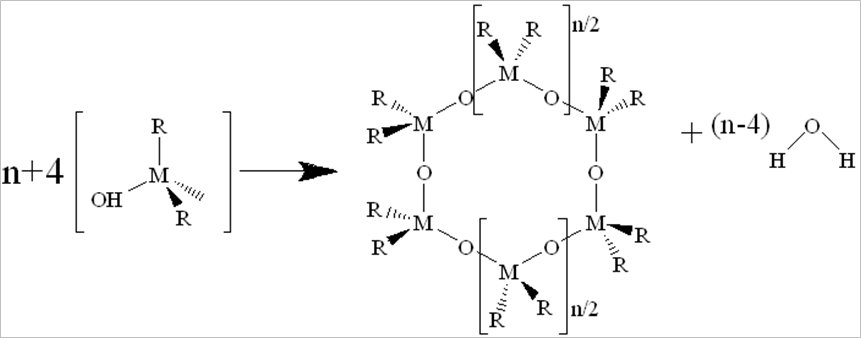
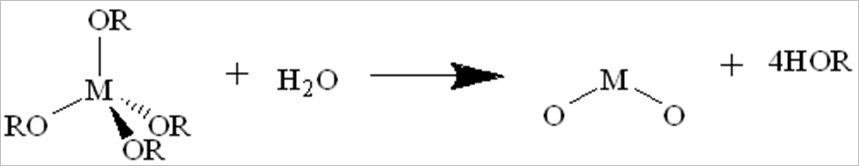
Porous materials used for shale gas storage: A feasible wet-chemistry was used to produce series shielded porous metal oxides and metal-organic frameworks for CH4 storage. The processing of condensation can be catalyzed by the addition of an acid or base in an excess of water. Its brief equation is shown as: 2M(OR)(4-n)(OH)n ® (M(OR)(4-n)(OH)(n-1))2O + H2O. This process allows the small molecules (water or alcohol) to be liberated, further to build larger molecules. The large molecules formation occurs through polymerization, formation of dimer, chain and ring, followed by gelation (as shown in Eqs. 1 to 4).
Eq. 1. Dimer formation:
Eq. 2. Chain formation:
Eq. 3. Ring formation:
Eq. 4. Condensation reaction to form gel:
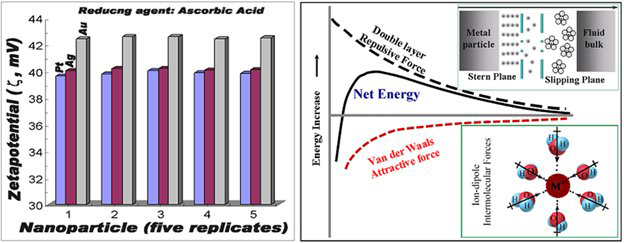
Fig. 1: The wet-chemistry was used to produce porous materials: the stability of the colloidal system due to the double layer repulsive forces relative to attractive van der Waals forces.
The synthesis method variables were optimized to control the porosity, size and shape of nanocomposites. Particles agglomeration was prevented by incorporation of a surfactant capable to improving the dispersion of the final product. This process has the added advantage of improving the wetting and dispersion of the surface of the engineering nanoparticles (ENPs). To maintain the stability of the colloidal system, the double layer repulsive forces must be dominant, relative to attractive van der Waals forces resulting from ion-dipole moments between the noble metal ions and H2O molecules (Fig. 1). Dispersing agents, such as gum Arabic (GA) polymers are adsorbed onto the particle surface and cause van der Waals forces weakening. Significant steric repulsion prevents nanoparticles (NPs) from being adhered and the particle surfaces are separated as a result.
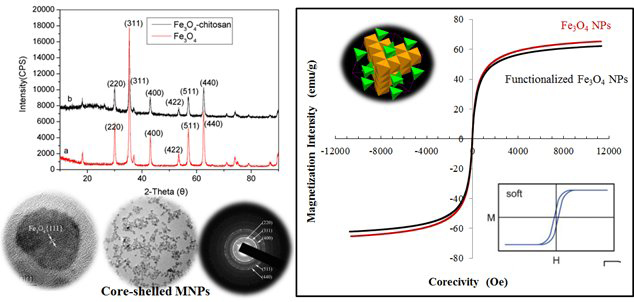
Fig. 2: Previous results of Fe3O4 NPs (selected as demonstration).
Materials characterization: Those so-prepared materials were characterized using the state-of-the-art techniques (TEM, XRD, XPS and BET) to evaluate their nanostructure, porosity and gas uptake. The materials safety was also evaluated by measurement of toxicology with a focus on the cellular assays to determine cellular homeostasis developed using ultrafine particles. The morphology and elemental composition of the nanocomposite were characterized using a transmission electron microscopy (TEM) (FEI Company, Tecnai F20-G2, Hillsboro, OR). The TEM images of three series nanocomposites indicate that the shapes of these fabricated materials (totally 48 formulations were prepared) were controlled as rod-shaped crystals with an average diameter at 10 nm and length at 78 nm, and fibre depending on the shielding agents and heat-treatment conditions. The powder X-ray diffraction (PXRD, Bruker-AXS D8 Vario X-ray Powder Diffractometer, Madison, WI) study indicated that nanocomposites and Ag-MOFs were highly crystalline. Briefly, the nanocomposites were generated by a colloidal chemistry approach to produce well-defined and highly crystallized compounds. The nucleation of the crystal units was found to be promoted when the solvent molecules were evaporated from the solution. This controllable nucleation favored the formation of composites with tunable size and monodispersity. The natural product extracts play a critical role in the shape of the nanocomposites.
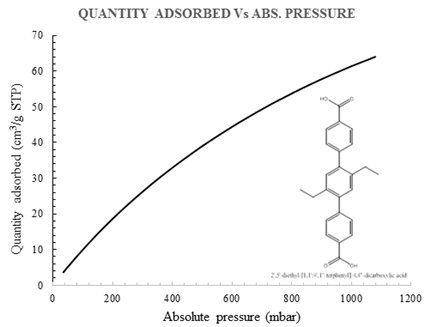
Fig. 3: CH4 adsorption isotherm of selected porous materials (Data collected by Xuan Wang and Uchenna Okakpu).
Gas uptake (CH4): The BET absorption model (Micromeritics ASAP 2020, Norcross, GA) and related measurement techniques will be applied. The gas of CH4 was used to evaluate the catalyst capacity for gas selective adsorption. The measurement temperatures are controlled at 87 K, 175 K, and 231 K using liquid argon, methanol-liquid nitrogen, and acetonitrile-dry ice bath, respectively. Before adsorption measurement, the samples will be activated using the “outgas” function of the adsorption analyzer for 12 hours at 80 °C. Then these activated samples will be used in gas adsorption measurement in the presence of purity grade. One of the selected porous materials (MOF XW2) shows an impressive methane uptake (Fig. 3). It is estimated that the isoreticular metal-organic framework motif in the XW series will show higher uptake and higher surface area with the presence of the extended side aliphatic chains.
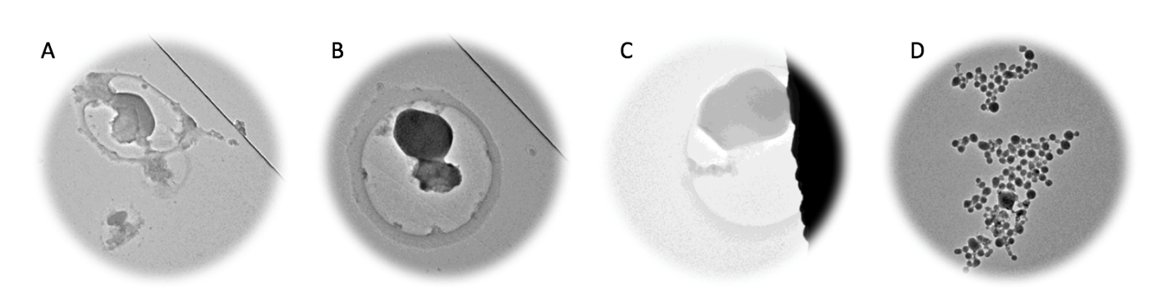
Materials Safety Evaluation: The prepared porous materials used for CH4 capture were tested using Retinal Pigmented Epithelium (RPE). The NO release from the cell when was attacked or contacted to the pollutes, however nitric oxide release is invisible so the NO indicator was developed to see the NO release. DAF-FM and DAF-FM diacetate are two critical nitro oxide indicators; these compounds are essentially non-fluorescent until they react with NO to form a fluorescent benzotriazole derivative. The cell-permeant characteristic of DAF-FM diacetate induced this kind of dye passively diffuses across cellular membranes. The intracellular esterases deacetylated DAF-FM diacetate to become DAF-FM.
Fig. 4: TEM image of damaged RPE whole cells; D: the whole cell after fixation with glutaraldehyde (3 vol % in DMSO, TEM data collected by Hsuan-Yi Huang and Jialuo Li).