Reports: DNI555900-DNI5: Targeted Size Mesoporous Zeolites for Deep Desulfurization of Petroleum Streams via Adsorption
Ioulia Valla, PhD, University of Connecticut
The biggest challenge for the deep-desulfurization of transportation fuels is the heavy, refractory sulfur compounds, such as Benzothiophene (BT) and Dibenzothiophene (DBT). The presence of aromatic compounds in fuels makes selective removal of the sulfur compounds difficult. During last year we found that the mesopores shorten the diffusion path length toward the internal active sites of the zeolite, while the metals (Ce and Cu) enhance adsorption. That work, however, did not study the desulfurization of liquid fuels in the presence of other aromatic compounds. This year we introduced bimetals (Ce and Cu) into our mesoporous Y zeolites (SAY) to further enhance the selective adsorption of sulfur compounds from a model fuel mixture containing aromatics and we studies the mechanism of adsorption. To the best of our knowledge, there are no studies on the adsorptive desulfurization performance of bimetal-exchanged mesoporous Y zeolites in a mixture of aromatics. Hence, the current results highlight the role of metals, bimetals (Cu and Ce) and mesoporosity in Y zeolites on the desulfurization performance of model fuels, containing BT and DBT.
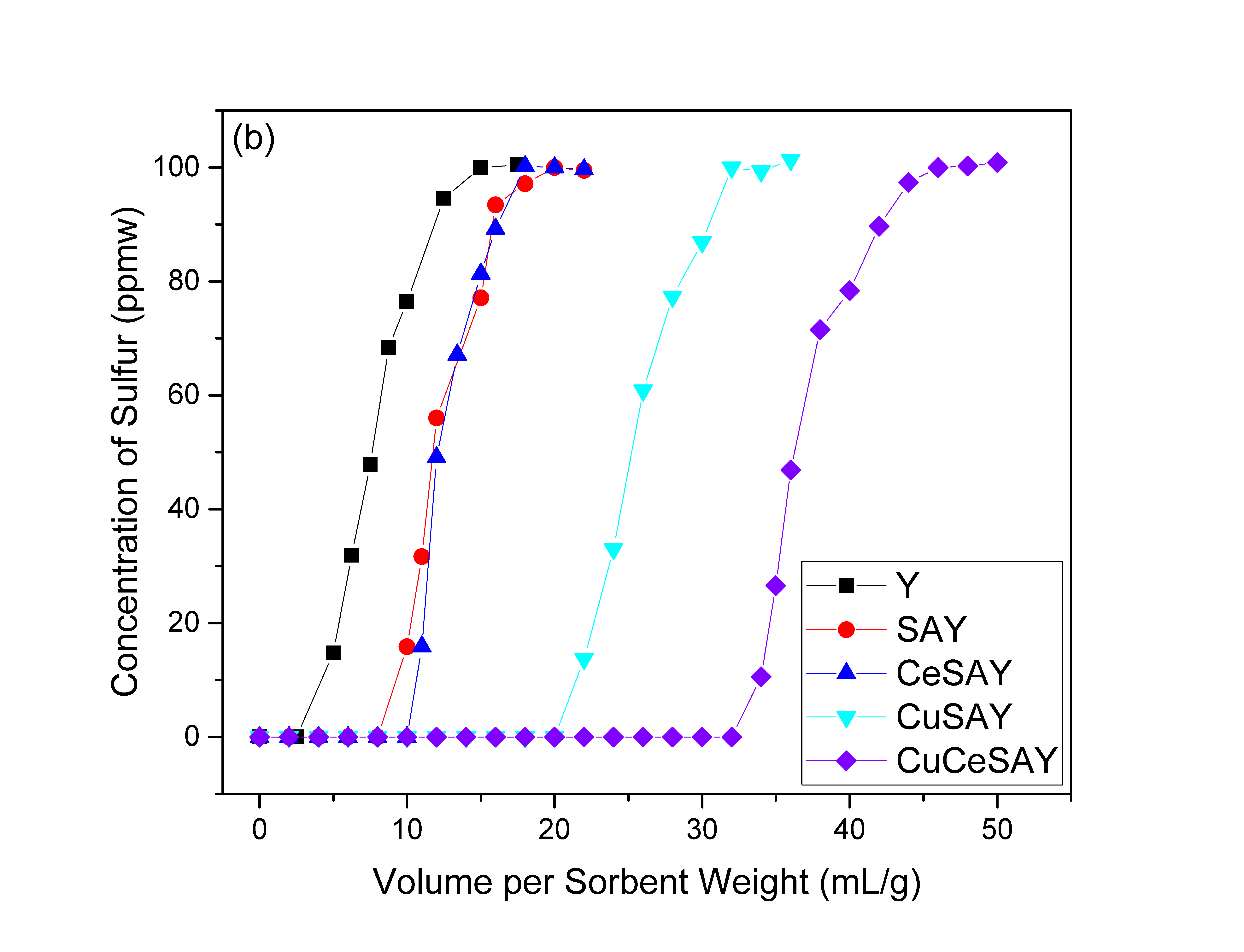
Figure 1 shows the breakthrough curves of BT and DBT in a mixture of 80% n-octane and 20% benzene. Figure 1(a) shows that BT breaks through very early on Y and reaches saturation within 5 mL/g of feed. As Ce cations were incorporated in the Y zeolite, the breakthrough point was extended from 0.5 mL/g to 8.5 mL/g indicating an increase in the adsorption of BT. CuY showed an even higher adsorption capacity of about 12 mL/g. When both Ce and Cu cations were used in the Y zeolite, the ability to adsorb BT increased even more to 15 mL/g of sulfur-free fuel. We also studied the desulfurization of DBT in the presence of benzene (Figure 1(b)). Similar to BT, the adsorption capacity of the parent Y is very poor. To overcome the aforementioned challenges, mesoporous Y developed (SAY) was used as a sorbent, and it successfully produced twice the amount of DBT-free effluent compared to parent Y. The addition of Ce cations to the mesoporous SAY did not improve the desulfurization performance, most likely due to the allocation of Ce cations on sites that are inaccessible for DBT to enter. CuSAY, on the other hand, showed remarkable improvement in the adsorption capacity of DBT, as the addition of Cu cations extended the breakthrough point from 10 mL/g to 20 mL/g of clean fuels. Furthermore, as observed in Figure 1(b), CuCeSAY dramatically increases the adsorption capacity of DBT to more than 30 mL/g of clean fuel. This suggest that the synergistic interaction between Ce and Cu metals plays an important role in the selective adsorption of refractory sulfur compounds, while the mesoporosity yields higher bulk mass transfer to the internal active sites.
Figure 1: Breakthrough curves of (a) BT and (b) DBT in a mixture of 80% n-octane and 20% benzene on various Y zeolite adsorbents.
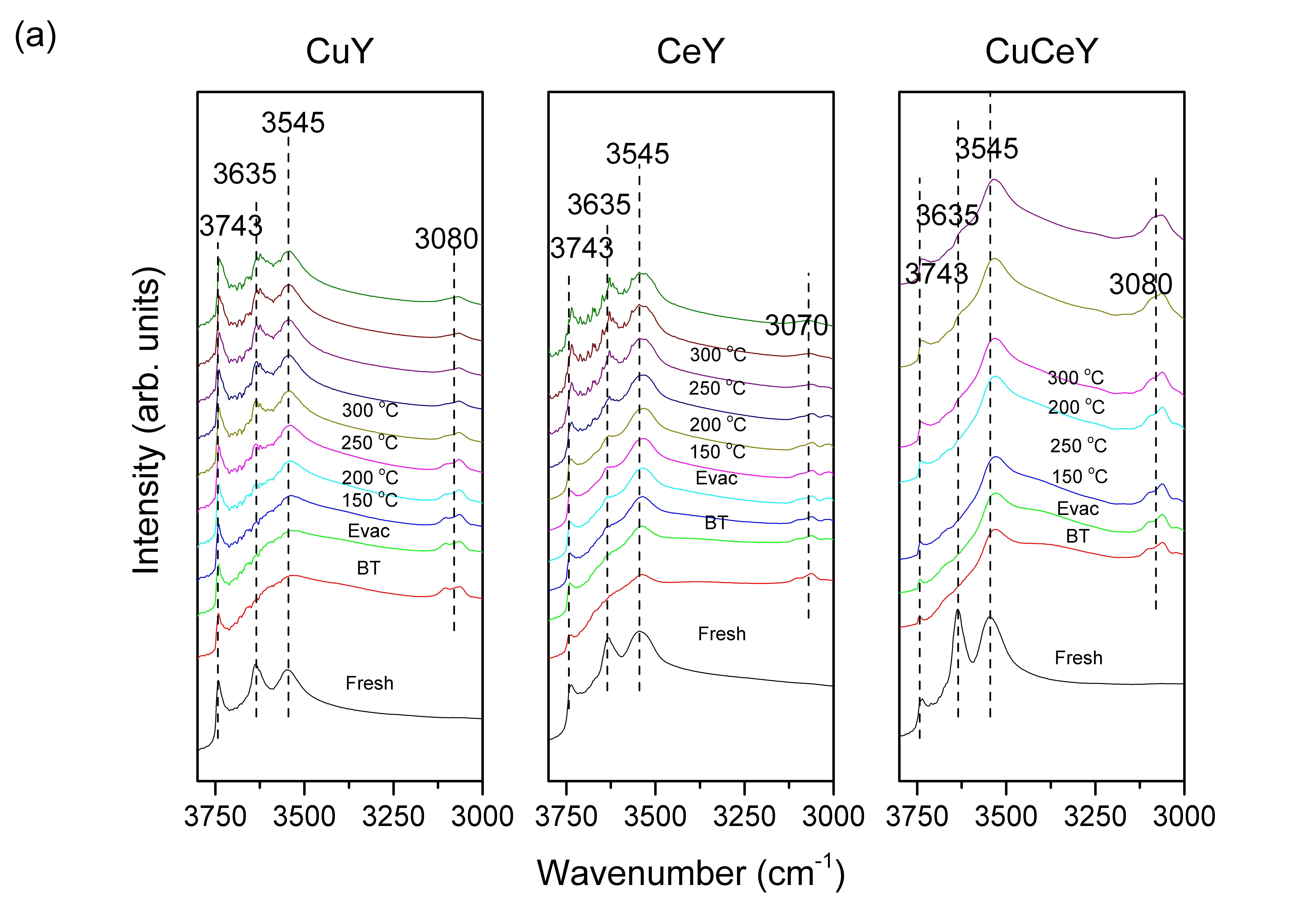
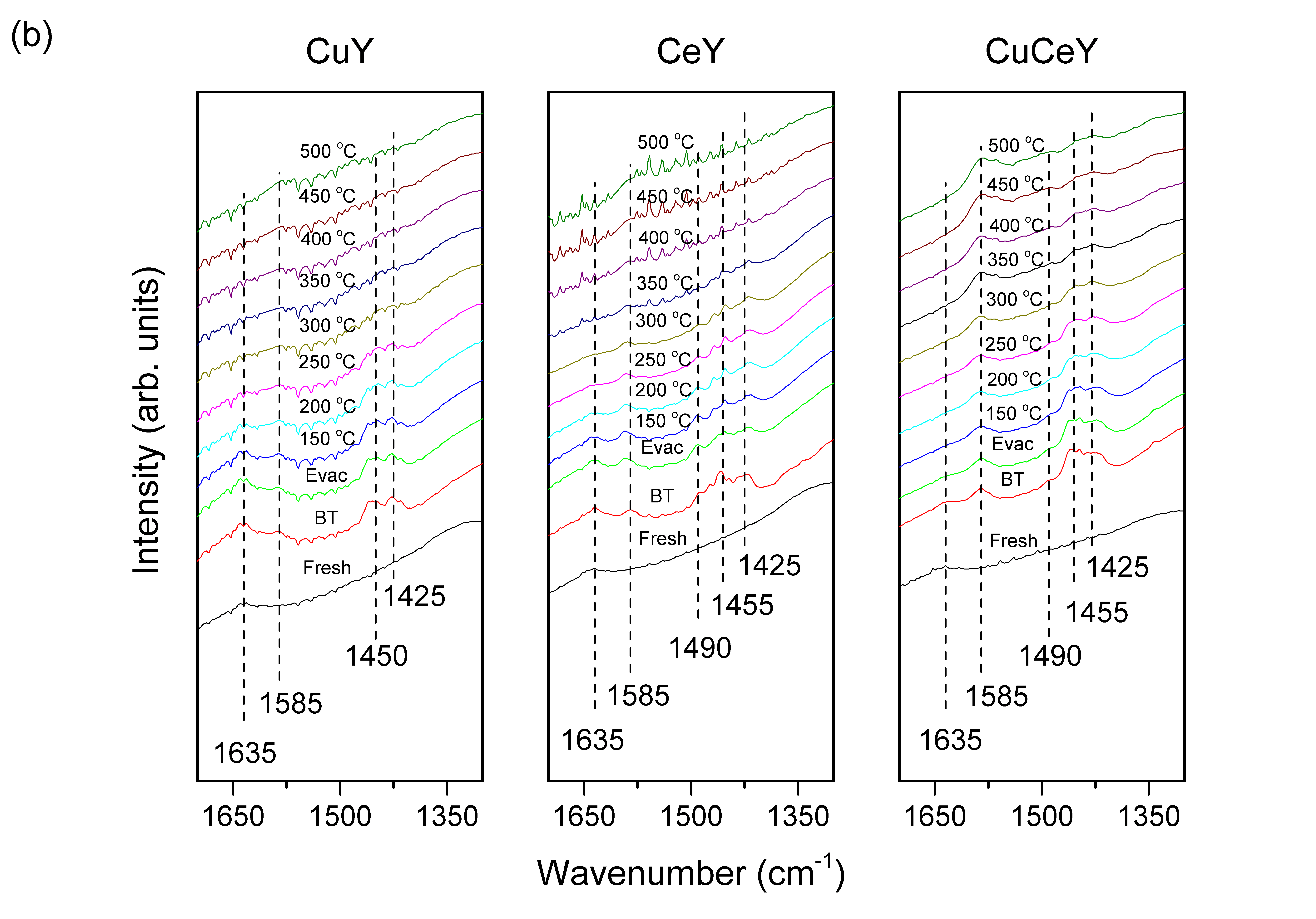
To better understand the adsorption behavior of the studied sulfur compounds, in-situ DRIFTS experiments were performed. Figure 2 shows the DRIFT spectra of BT on the tested materials recorded from Temperature Program Desorption (TPD) under vacuum. Vibrational peaks at 3500 to 3750cm-1 are characteristic of the hydroxyl-region. The peak at 3743cm-1 is due to terminal SiOH, while peaks at 3545 and 3635cm-1 are due to hydroxyl-groups in framework. Any peaks above 3000cm-1, are due to vibrational stretches of C-H bond of the thiophenic ring. Peaks ranging from 1600 to 1300cm-1 are mostly due to vibrational stretches of C=C bond of the thiophenic ring. Figure 2(a) shows that the hydroxyl-sites of CuY were occupied rapidly upon exposure to BT vapor. The peak located at 3080cm-1 is due to C-H of the BT ring, which confirms adsorption. By subjecting the sample to TPD the physisorbed BT vapor was removed. The adsorption of BT on CuY triggers vibrational stretches and bending of C=C bonds of the five-membered ring that show up in regions below 1700cm-1 (Figure 2(b)). As CuY undergo TPD, vibrational peaks at 1425, 1450, 1585 and 1635 cm-1 were observed. Free BT exhibits a peak at 3080 cm-1 which is due to C-H vibration, while the peak at 1450cm-1 corresponds to symmetrical C=C vibrations. Upon adsorption on the surface, a shift in the frequency of vibration is expected due to change in electron density. As a result, the peak due to free BT can shift to higher or lower wavenumbers. The peak at 1425 cm-1 might be attributed to the symmetrical C=C stretching vibration of free BT, which has been shifted to lower wavenumber. The red shift of the C=C stretching peak suggests an elongation of the ring due to electron density reduction, which occur when BT ring is adsorbed on Cu via π-complexation. CeY exhibits the same IR peaks generated on CuY with the addition of a peak at 1490cm-1. The appearance of this peak at higher wavenumber relative to free BT can be ascribed to an increase in electron density of the BT ring, implying that the adsorption of BT on Ce occurs via direct σ-bond interaction. These results suggest that Ce can adsorb aromatic sulfur compounds via two types of adsorption modes, which makes it highly beneficial for selective adsorption of sulfur compounds in a mixture of aromatics. Figure 2(b) also shows that the IR spectra of CuCeY exhibit similar trends of the corresponding metal-exchanged parent Y zeolites. These include vibrational peaks at 1425 and 1490cm-1 which are resulted from π-complexation and σ-bond interaction of BT and CeCuY zeolite. Interestingly, we report that in the case of CuCeY, the 3635cm-1 vibrational band is not recovered even at 500°C. This observation suggests that higher temperature are required to completely desorb BT from the surface, which in turn underscores a much stronger interaction.
Figure 2: FTIR spectra during BT adsorption and temperature-programmed desorption on CuY and CeY of the (a) zeolitic region and the (b) sulfuric C=C region
DRIFTS experiments were performed using DBT adsorption and the results were similar with the corresponding DRIFTS for the BT.