Reports: UNI356702-UNI3: Bisguanidine Complexes of Pd(0) and Ni(0) Towards Catalytic Removal of Aryl-heteroatoms From Heavy Crudes
Brian M. Barry, PhD, University of Wisconsin, Platteville
Proposal Overview
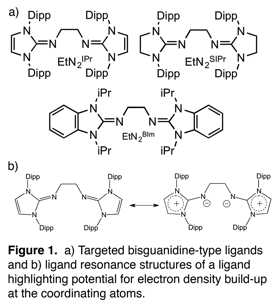
We proposed the development of Ni0 and Pd0 complexes supported by bulky, neutral bisguanidine-type ligands capable of enhanced electron-donating ability via cation stabilization in the imidazolium ring and concurrent transfer of electron density to exocyclic nitrogen atoms (See Figure 1b). These complexes would then be used to perform oxidative additions (OAs) across various otherwise inert sp2-heteroatom (N, O or S) bonds found in asphaltenes, and following OA a reductive elimination step would result in the removal of the heteroatom and regenerate the metal complex in catalytic fashion. Our catalyst design proposals were aimed at producing a complex with a particularly electron-rich metal center while also producing a coordinatively unsaturated metal center. The combination of these attributes were thought to, as advised by literature and our inclinations, give the catalyst the best shot at performing an OA, or insertion, across the targeted inert bond. In order to achieve these attributes in the resulting complex, the ligand must have excellent electron-donating ability (electron-rich metal center) and provide steric protection (coordinatively unsaturated metal center). The majority of the funding in year one has been used to accomplish the first major hurdle in the project-- ligand production. Other work in the first year has included probing the coordinating ability/nature of the ligands as well as probing its electron donating capabilities through both experimentation and computations which have just started to get underway.
Ligand Production
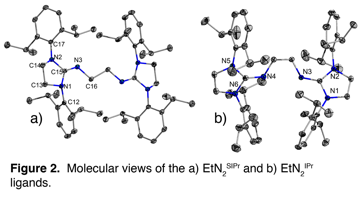
The development of reproducible synthetic pathways leading to highly pure ligands in manageable yields was no small undertaking as they were 7-step processes which provided lots of opportunities for unwanted chemistry. In addition, many of the steps were found to require multi-day reaction durations, the last reduction step for instance, was found to require heating in refluxing toluene for no less than 7 days to achieve a complete reaction. Of the three proposed bisguanidine ligands we aimed to target, we successfully produced both the EtN2IPr and EtN2SIPr ligands and were able to crystallographically characterize them (See Figure 2).
Complex formation
We proposed to target Pd0 and Ni0 complexes by reacting the bisguanidine-type ligands with various molecular, zero-valent Ni and Pd reagents such as Ni(COD)2 or Pd(dba)2 (COD= 1,5-cyclooactadiene; dba = dibenzylideneacetone) as we imagined the COD and dba ligands could be easily replaced by our ligands. Several attempts were made under various reaction conditions, but have not been successful to date. When cold temperatures (-78 °C to room temperature) were used no reaction took place and upon heating the reaction resulted the formation of black, insoluble powders, presumably metal powders. Perhaps there is a sweet spot using gentle heating and the right duration, but we have not found it yet. These targets have been shelved for the moment and other targets are in pursuit.
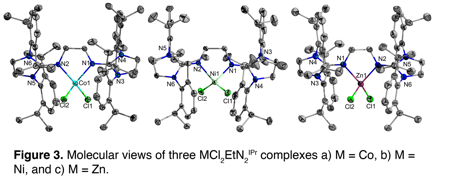
We were quite surprised in the lack of coordination of our ligands to the zero-valent metal centers which motivated us to simply see if we could coordinate our ligands to a metal center at all, after all the ligands are quite bulky. Or perhaps it's the contrary--the envisioned 2-coordinate, zero-valent metal centers are not sterically protected enough and the resulting complex behaves as a coulombic vacuum. The next experiments simply targeted the ligand coordination to various divalent transition-metal chlorides. This proved to be fruitful and 3 complexes (MCl2EtN2IPr, where M = Co, Ni or Zn) were successfully produced and X-ray quality crystals afforded crystal structures of the three complexes (See Figure 3). In all 3 compounds a highly distorted tetrahedral coordination environment around the metal-center resulted. It should also be noted that analogous reactions targeting the PdCl2 analog could not be afforded, likely due to the ligands being too bulky to allow for a square planar complex which would be expected from a Pd(II) metal center.
New 2-coordinate Targets
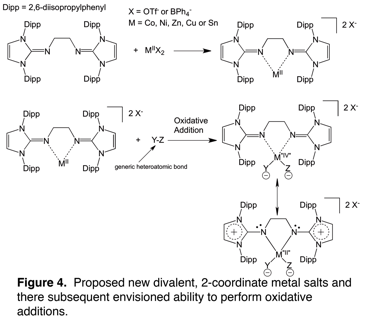
As the Ni0 and Pd0 complex syntheses were unsuccessful so far, we thought it appropriate to think of ways to target alternative 2-coordinate (coordinatively unsaturated) complexes. To this end, we envisioned the ligand reacting with a divalent metal with weakly coordinating anions such as triflates or tetraphenylborates. Upon ligand coordination, it is our hope that the anions are displaced from the metal center producing a 2-coordinate salt (See Figure 4). Attempts to attain crystal structures of two such complexes ([MEtN2IPr][OTf]2, where M = Sn or Zn and OTf = triflate) have just begun and our spectroscopic data is consistent with the targeted complexes. Figure 4 also shows how the salts are proposed to be used in a generic oxidative addition reaction highlighting the potential flexibility in electron-donating ability of the ligand accommodating the metal center as its oxidation state fluctuates throughout a catalytic cycle. Producing and characterizing these complexes, along with testing their reactivity with various substrates, will be the primary focus of the research moving forward so long as the results continue to be promising.
Computational Work
Density functional theory and NICS (nucleus-independent chemical shifts) computations have very recently started to be performed using atom locations produced from various crystal structures. The hope is that the computations can give some idea how well the ligands are doing at donating electron-density and also to see if the aromatization of the imidazolium ring can be correlated to states in which we see increased electron densities around the metal center. Furthermore, monoprotonated and diprotonated ligands ([EtN2HIPr][OTf] and [EtN2H2][OTf]2) have been successfully synthesized and crystallographically characterized to gain further insights into the ligands behavior upon coordinating to cationic species. Initial results form NICS calculations indicate that the ligands do in fact result in the imidazolium rings showing increased aromaticity relative to the neutral (unprotonated) analogs, these NICS values will be used as a reference point for comparison purposes when NICS values are calculated in the imidazolium rings of the metal complexes.