Reports: ND754047-ND7: Elucidate the Molecular Orgins of Mechanical Stress in Large Deformations of Polymer Glasses by Incorporating Chromophores into the Backbone as Molecular Probes
Abraham Joy, The University of Akron
Shi-Qing Wang, University of Akron
Polymer glasses are promising materials for its wide application in various areas because of its good mechanical properties, light weight, low cost, optical transparency and reusability. Yet some of those glasses suffering brittle facture and lost the ability to yield and undergo large deformation, which limit its application. It is of fundamental and practical importance to study the mechanical behaviors of polymer glasses at a molecular level. One of the key feature is that polymeric glasses of high enough molecular weight (MW) exhibit brittle-to-ductile transition (BDT) when altering the temperature and rate of deformation. In the past decades, the Ludwik−Davidenov−Wittman−Orowan (LDWO) hypothesis regard yielding and brittle fracture as two independent, competing process. Wu suggested the secondary relaxation behavior to be correlated with BDT [1], while Kramer studied how entanglement density controlled crazing behavior. Despite those efforts summarized in many publications, a coherent zero-order molecular theory for yielding and BDT is still incomplete.
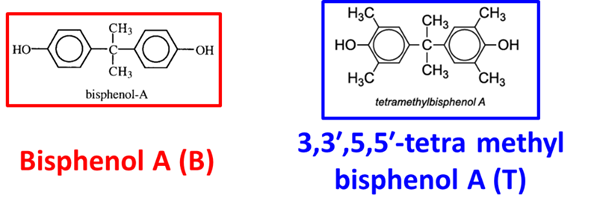
As we know, bisphenol A polycarbonate (BPA-PC) is one of the most ductile glassy polymer that could undergo yielding, necking propagation and strain hardening before fracture at room temperature. Non-bisphenol A polycarbonates made by modifying the bisphenol phenylene rings, on the other hand, has been found to affect both the mechanical proprieties and glass transition temperature (Tg). Tetramethyl-Bisphenol A polycarbonate (TMBPA-PC), as shown in Figure 1, for example, is one in the non-bisphenol A PC class that has been extensively studied.
Figure 1 Chemical structures of bisphenol A and TMBPA polycarbonate
Specifically, two CH3- substituents to replace the H- on each aromatic ring produce a 3,3’,5,5’-tetra-substituted bisphenols repeating unit in the main chain, leading to an increased Tg as well as a higher rotation barrier. The increased Tg of TMBPA-PC would be a promising modification to expand the range of service temperature for the PC family as heat resistant and flame retardant material. However, the mechanical propriety of this specific PC turns out to be reduced, i.e., it shows brittle facture at room temperature.
To illustrate what controls BDT for all polymer glasses a theoretical picture must show how and why different molecular parameters control macroscopic ductility or brittleness of polymer glasses. From a practical standpoint, we need to understand why the TMBPA-PC turns brittle so that we might be able to revise the synthesis of ductile PC for high-temperature performance. The conventional wisdom correlates the polymer ductility with the γ relaxation (secondary relaxation) for the polycarbonate family, claiming that the activation of cooperative secondary relaxation of several repeat units is necessary for the yielding process. However, other evidence suggested that annealing has little effect on the γ relaxation but reduces ductility. In addition, unpublished results show that melt stretching hardly affects the secondary relaxation in polystyrene (PS) while turning it into a ductile glass. During the extension of the grant, we carried out synthesis and analyze to examine how and why two substituents on each aromatic ring in TMBPA-PC brings back brittleness at room temperature. We synthesized TMBPA-PC with molecular weight of 25k and PDI=1.6. Because of the steric hindrance during the polymerization, such a molecular weight is the highest we could achieve for the TMBPA-PC.
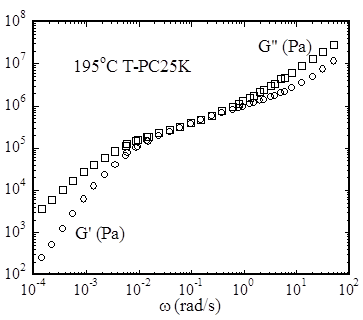
Figure 2 SAOS data.
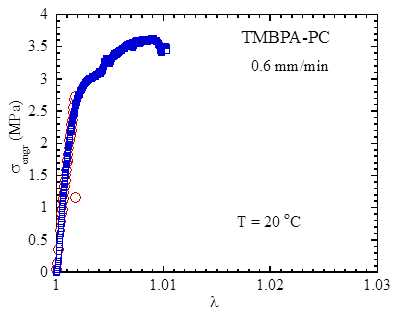
Figure 3 Stress vs. strain during extension at room temperature from two runs.
Linear viscoelastic spectrum was obtained using small amplitude oscillatory shear (SAOS) based on an ARES rotational rheometer. A master curve was constructed, as shown in Figure 2, according to time-temperature superposition principle from frequency sweep data in the temperature range from 195 to 210 oC. The SAOS data indicates that (a) TMBPA-PC has a rather low elastic melt plateau modulus, ca. 0.3 MPa, and corresponding a rather high entanglement molecular weight Me > 10 kg/mol and (b) the molecular weight of this sample is indeed rather low, and thus hardly entangled, which is not surprising given M/Me < 2.5.
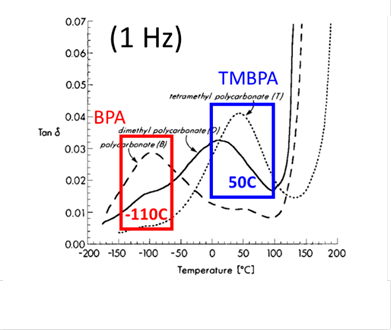
Figure 4 Dynamic mechanical analysis measurements of two PCs [2].
Tensile extension test was subsequently carried out, as shown in Figure 3, confirming that such a TMBPA-PC is completely brittle [3], unlike bisphenol A PC that is ductile at a comparable molecular weight [4]. In the past, the loss of ductility in TMBPA-PC was assumed to arise from the shift of the secondary relaxation transition to the much higher temperature as shown in Figure 4. Based on our measurements, we conclude that the reasons for the literature report of brittle fracture of TMBPA-PC are multiple, the least of which is that the secondary relaxation occurs at a much higher temperature in comparison to bisphenol A PC. The more pertinent factors are listed as follows. First, since the achievable molecular weight is so lower that chain network can hardly form in TMBPA-PC, it is inevitably brittle. Second, the chain network density is similar to that of PS and therefore is insufficiently high. According to our analysis [5], the areal density of effective strands in the network is inversely proportional to the packing length, which scales with Me with exponent 1/3. Because TMBPA-PC has a high value for Me, comparable to that of PS, TMBPA-PC can be expected to be brittle at room temperature for the same reason PS is brittle [5].
References
[1] Wu, S., ‘‘Secondary relaxation, brittle ductile transition-temperature, and chain structure,’’ J. Appl. Polym. Sci. 46, 619-624 (1992).
[2] Goetz, J. M., Wu, J., Yee, A. F., and Schaefer, J., ‘‘Bundle Description of Packing and Dynamics in Polycarbonate Homopolymers, Copolymers, and Blends,’’ Macromolecules 31, 3016-3020 (1998).
[3] Xiao, C., Jho, J. Y., and Yee, A. F., ‘‘Correlation between the Shear Yielding Behavior and Secondary Relaxations of Bisphenol A Polycarbonate and Related Copolymers,’’ Macromolecules 27, 2761-2768 (1994).
[4] Wu, J. H., Xiao, C. D., Yee, A. F., Klug, C. A., and Schaefer, J., ‘‘Controlling molecular mobility and ductile-brittle transitions of polycarbonate copolymers,’’ J. Polym. Sci. Pt. B-Polym. Phys. 39, 1730-1740 (2001).
[5] Wang, S.-Q., Cheng, S., Lin, P., and Li, X., ‘‘A phenomenological molecular model for yielding and brittle-ductile transition of polymer glasses,’’ J. Chem. Phys. 141, 094905 (2014).