Reports: DNI356562-DNI3: Metal-Free Nucleophilic Borylation Chemistry Using Boron Cluster Reagents
Alexander Spokoyny, PhD, University of California, Los Angeles
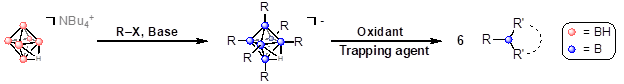
Scheme 1. Proposed method for nucleophilic functionalization of the hexaborate core followed by controlled oxidative degradation to form desired alkyboron species.
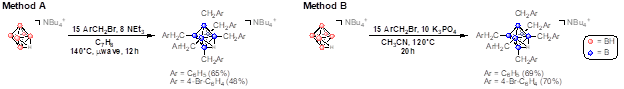
A microwave-based synthetic route to hexaborate clusters of the type [NBu4][B6(CH2Ar)6Hfac] (Ar = C6H5, 4-Br-C6H4) was established by our group in a recent communication (see attached reference). This establishes, for the first time, a synthetic route for substituting each boron vertex with a carbon-based electrophile. For the ultimate goal of oxidative degradation (vide infra), this is crucial for maximum boron atom economy. Furthermore, we have expanded this method to include thermal methods that do not require microwave heating, as well as an expanded electrophile scope for the formation of additional, perfunctionalized hexaborate species (Figure 1). The desired compounds can be isolated by precipitation in good yields.
Figure 1. Complementary synthetic methods for the formation of perfunctionalized hexaborate clusters with benzyl electrophiles.
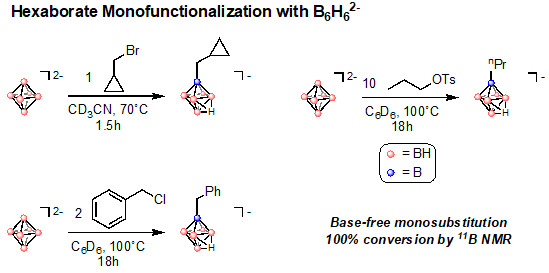
Given the forcing conditions for hexaborate perfunctionalization, we have also considered that certain electrophiles desirable for borylation may not be stable to high temperatures and long reaction times. We therefore set out to establish alternative protocols for single vertex functionalization under mild conditions. Expanding on previously established methodology, we find that monosubstitution is tolerant to potentially reactive groups such as cyclopropanes. These monosubstitution reactions proceed with electrophiles in a 1:1 ratio to the hexaborate nucleophile. The 11B NMR of the monosubsituted products provide diagnostic chemical shift patterns that indicate conversion to the desired product. Importantly, these substitutions are not limited to alkyl or benzylbromides and are in fact compatible with alkyl and benzylchlorides and tosylates (Figure 2). We are currently in the process of further expanding the electrophile scope for these monosubstitution reactions.
Figure 2. Electrophile scope for the monosubsitution of the hexaborate dianion. Alkyl bromides, chlorides, and tosylates all react to form monofunctionalized hexaborates.
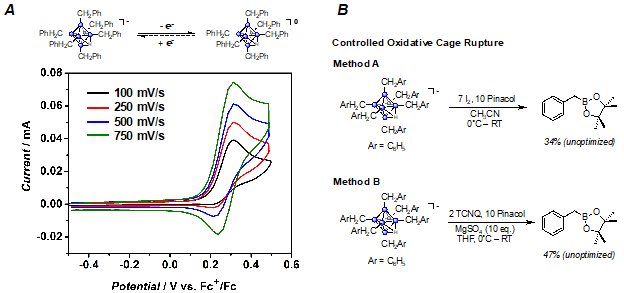
Ultimately, controlled cluster degradation in the presence of a trapping agent (e.g. pinacol) should furnish synthetically useful reagents such as alkylboron pinacol esters. The possibility of this goal is supported by the electrochemical behavior of the perfunctionalized clusters: irreversible oxidative waves are observed for both of our reported perfunctionalized derivatives (Figure 3A), suggesting that cage degradation is feasible via oxidation. We have found two synthetic routes that indicate formation of the desired alkylboron pinacol esters. In particular, treatment of [NBu4][B6R6Hfac] with either I2 or tetracyanoquinodimethane (TCNQ) in the presence of base and pinacol furnishes new products consistent with the formation of the desired compounds as judged 1H and 11B NMR spectroscopy and mass spectrometry (Figure 3B). We are currently in the process of optimizing the isolation protocols for these compounds and look forward to extending these oxidation procedures to the monofunctionalized hexaborate clusters (vide supra).
Figure 3. A) Electrochemical irreversibility of the benzyl-substituted hexaborate suggested accessible cluster degradation pathway. B) Complementary methods for controlled oxidative cage degradation using different oxidants to form desired alkylboron pinacol esters. Yields are unoptimized.
Overall, this method provides an alternative route to traditional borylation methods, many of which require transition metal catalysis. With the support of this grant in the upcoming year, we will expand this borylation methodology based on the encouraging leads disclosed above and further explore the potentially interesting chemical and electrochemical properties of the alkylated hexaborate species. We will also strive to develop a general method by which hexaborate–Csp2 bonds may be formed, further expanding the classes of organic molecules that can be valorized with boron-based functional groups.