Reports: UNI156161-UNI1: New Methods for the Synthesis of Spirocycles via Lewis Acid Catalyzed Dearomative Arene Alkyne Coupling
Paul Vadola, PhD, DePaul University
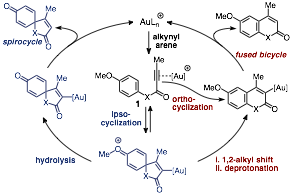
In the last year my group has continued its work on the development of arene-alkyne coupling methods for the synthesis of spirocyclic and fused bicyclic heterocycles (scheme 1). We previously developed an efficient method for the synthesis of spirocyclic lactones from aryl alkynoate esters gold catalysis (scheme 1, X = O), which served as the preliminary data for my initial ACS-PRF application. Follow the award we began to investigate the amide analogs of these substrates, N-aryl alkynamides, as a means of synthesizing spirocyclic lactams (scheme 1 X = N).
Scheme 1. Mechanism of spirocycle versus fused bicycle formation
Our initial investigations into this area led to the development of a highly efficient method for the synthesis of the fused bicycles 2-quinolinones, via ortho-cyclization of the pendant alkyne unto the arene core. This work was published in the Journal of Organic Chemistry in early 2017. More recently our efforts have focused on identifying a method to catalyze the ipso-cyclization of these substrates, which would grant access to the spirocyclic lactam framework.
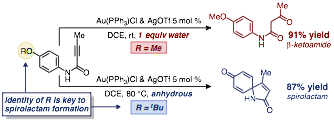
Scheme 2. The critical role of the p-substituent in the spirocyclization of N-aryl alkynamides
While our previously reported ester ipso-cyclization conditions failed to deliver the desired spirolactams, even at elevated temperatures, additional investigations into the mechanism of spirolactone formation, revealed that fine tuning of the arene para-substituent has a dramatic effect on the reactivity of the ester substrates, and also enables the ipso-cyclization of amides to yield spirolactams (scheme 2). We found that, while a p-methoxy group was critical for the ester substrates, p-methoxy aryl alkynamides undergo nearly quantitative hydration of the alkyne under our lead conditions (1 equivalent of water and AuPPh3OTf as catalyst) to give beta-ketoamide products. Running the reaction under anhydrous conditions, however, lead to varying degrees of undesired 2-quinolinone formation via ortho-cyclization, with trace amounts of the spirocycle arising from ipso-cyclization being observed.
In our original report, we suggested that the initial cyclization event of our ester substrates led to a spirocyclic intermediate containing an oxonium ion in the form of an O-methylated cyclohexadienone core, following dearomatization of the p-methoxy arene ring (scheme 1). In this study, we hypothesized that if we could increase the lability of the C-O bond we might be able to trigger heterolytic cleavage of the oxonium C-O bond to liberate the neutralized spirocycle and a carbocationic byproduct. Towards this end we screened a number of p-alkoxy aryl alkynamides and found that a tert-butyl group on the p-oxygen led to selective formation of the desired spirolactams in good yields and reasonable reaction times.
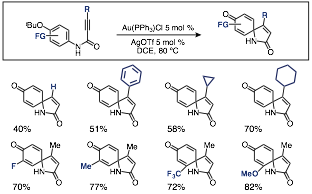
We found that an array of functional groups could be tolerated around the arene core, including electron-donating and –withdrawing groups, such as methoxy, fluoro, and trifluoromethyl groups. In addition, ortho-substituents did not inhibit the cyclization. However, we viewed the necessity of the p-tert-butoxy group as an undesirable structural requirement due to the limitations it placed on substrate synthesis.
Scheme 3. Selected p-tert-butoxy substrate cyclizations
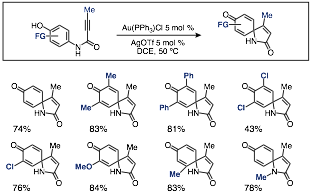
This caused us to turn our attention to p-hydroxy containing substrates (scheme 4). Unsurprisingly, these substrates cyclize very rapidly to give the same spirolactams in good yields. They are, however, fairly insoluble in common lab solvents and thus the reactions require heating between 50-80 ºC to ensure complete solvation and full conversion of the substrate. Nonetheless, given the large number of commercially available p-hydroxyaniline substrate precursors, we were able to expand the library of spirolactams to which our methods grants access.
Scheme 4. Selected p-hydroxy substrate cyclizations
This work is currently in the final stages of preparation for submission to the Journal of Organic Chemistry. I am thankful to the ACS-PRF for their continued support on this, and prior, project. During the course of these studies I have mentored nine undergraduate students in my lab, two of which have gone on to pursue their PhD in chemistry. Another three of those nine are currently applying to graduate schools to study organic synthesis specifically. Without this grant, much of this work would not have been possible.