Reports: DNI955591-DNI9: Investigating the Role of Wettability in Enhancing Shale Gas Production
Brian R. Ellis, PhD, University of Michigan
The overall goal for this project was to investigate the role of mixed surfactant fracturing fluids on the water loss and associated natural gas production after hydraulic fracturing of low permeability shale reservoirs. The first phase of this project investigated the effect of two commonly used surfactants, cationic n-octadecyltrimethyl ammonium chloride (OTAC) and anionic ammonium dodecyl sulfate (ADS), on the wettability alteration of Marcellus and Collingwood shale rocks using contact angle and surface tension measurements. This work demonstrated how a 1:1 mixture of ADS/OTAC could alter shale wettability from water wet to more oil wet at concentrations below the critical micelle concentration (CMC). Such rapid surface coverage by the mixed surfactant solution at low concentrations is aided by the mixed charge nature of the shale surfaces and could result in reduced capillary uptake of water in shale fractures. The second phase of this project examined the effect of ADS/OTAC 1:1 mixed surfactant adsorption on the water uptake rate in Marcellus shale using neutron imaging. These experimental efforts helped to elucidate the influence of surfactants in fracturing fluids on natural gas relative permeability and associated gas recovery during hydraulic fracturing.
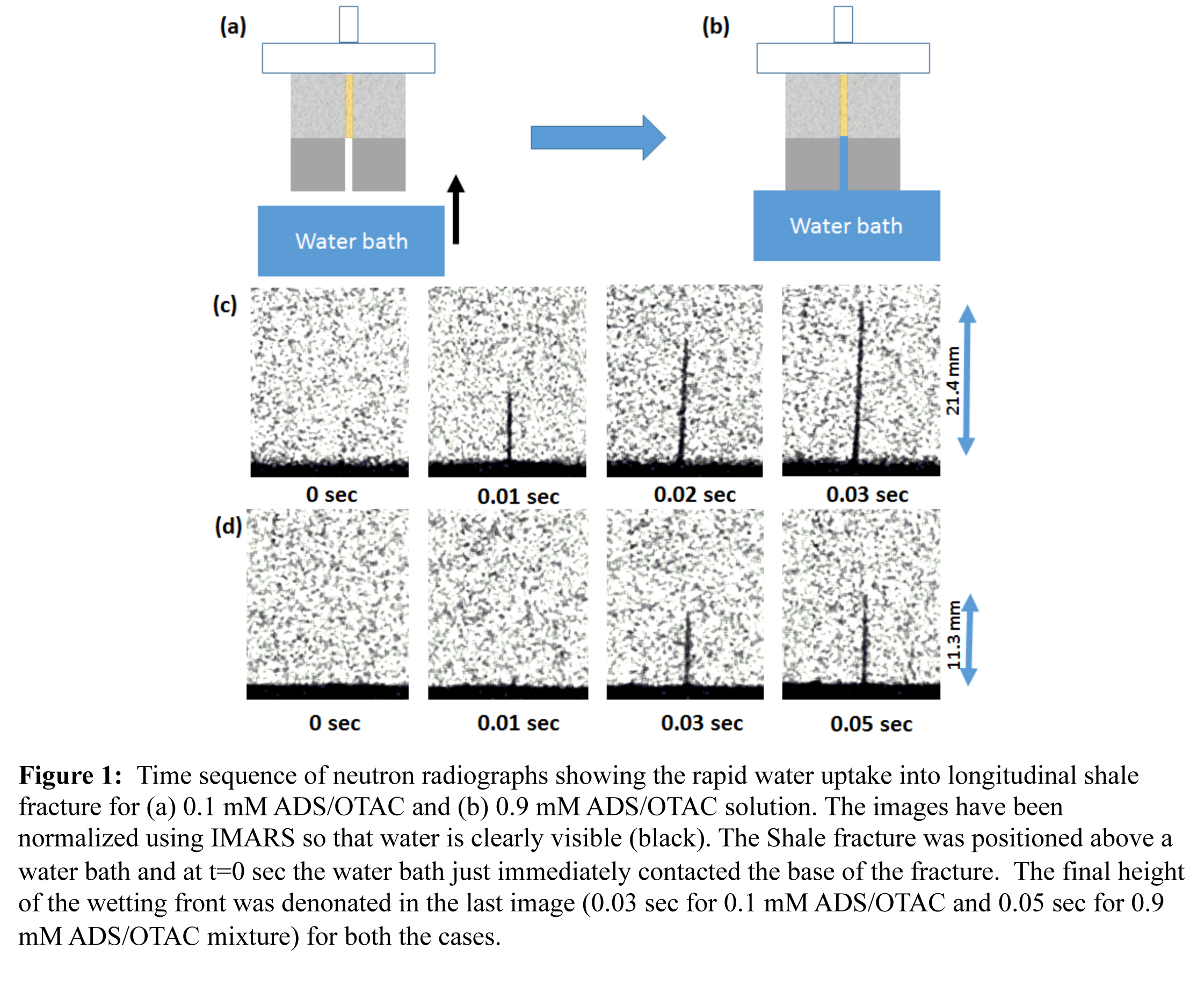
Neutron imaging was used to measure changes in the rate of water uptake into shale fractures under varying surfactant loading conditions. A simulated fracture was created by binding two hand polished rectangular slices of Marcellus shale separated by stainless steel spacers (100, 200, and 800 μm) at the top. The interface between the two shale surfaces mimics a longitudinal fracture with constant aperture equal to that of the spacer thickness (see Figure 1(a)). Two types of shale fractures were used for this study: (i) pre-exposed samples (samples soaked in ADS/OTAC mixture for 2 hours) designed to resemble shale surface conditions after well shut-in and (ii) unexposed shale samples resembling fracture surfaces encountered immediately after fracturing. The samples were mounted in front of the neutron beam and the water table was lifted until the reservoir touched the base of the shale fracture (Figure 1(b)). Figure 1(c) and 1(d) show the evolution of the water front along 200 μm wide air-filled Marcellus shale fractures through a series of neutron radiographs for 0.1 mM and 0.9 mM ADS/OTAC 1:1 mixtures, respectively. The neutron radiographs were normalized by the initial dry image so that water is clearly visible (black). At time t = 0 sec the water bath just touches the base of the fracture and the water begins to penetrate the fracture due to capillary suction. Subsequent images follow the water front as it moves upward into the fracture over time. As is evident in Figure 1, the water front reached the top of the fracture (21.4 mm) within 0.03 sec for the 0.1 mM ADS/OTAC 1:1 solution whereas the 0.9 mM ADS/OTAC 1:1 solution only rose to a height of 11.3 mm in 0.05 sec. The water front for the 0.9 mM ADS/OTAC experiment was followed for another 20 sec but there was no further rise into the fracture. The results clearly demonstrate that the water imbibition was slower for 0.9 mM ADS/OTAC mixture compared to the 0.1 mM ADS/OTAC mixture.
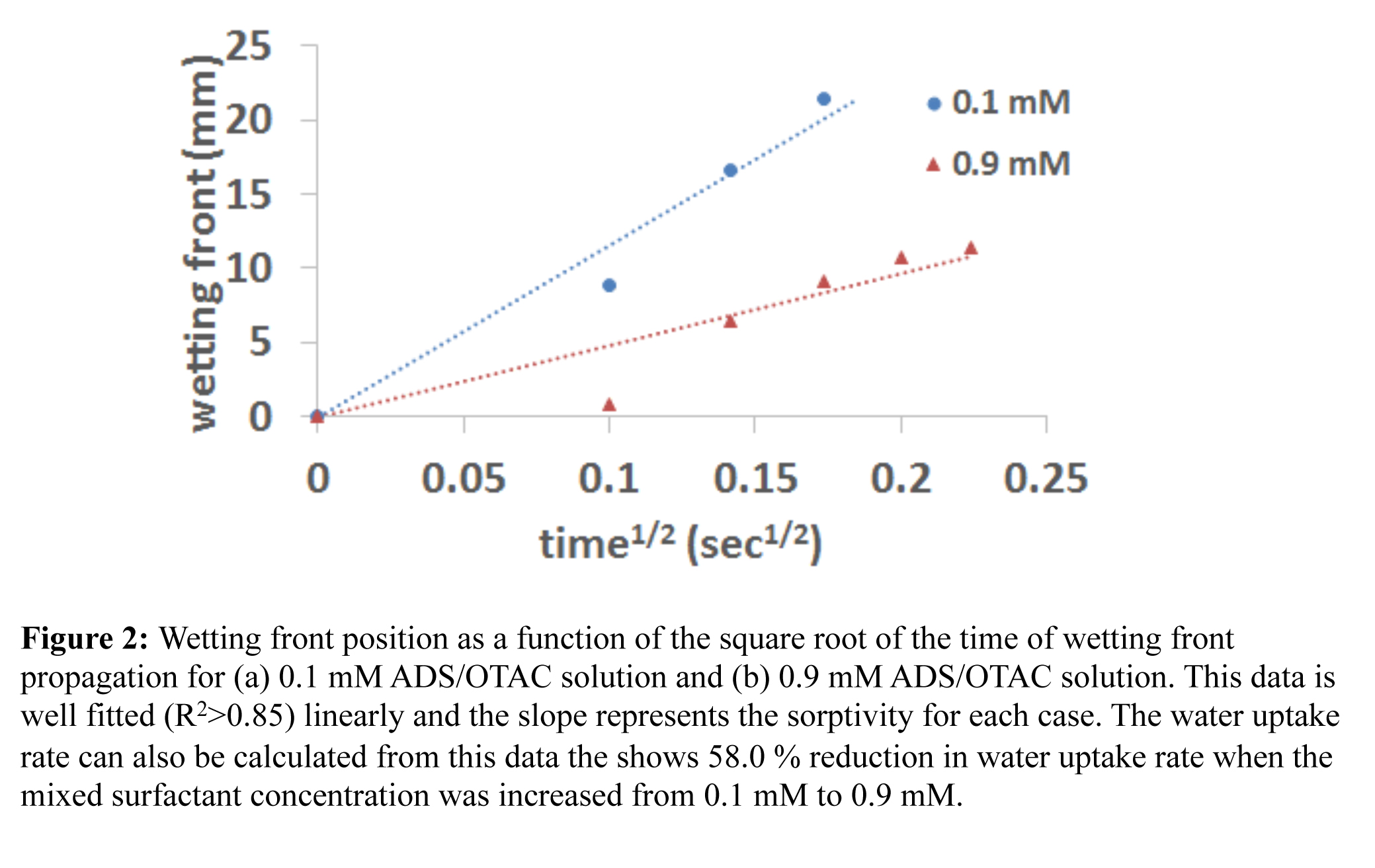
The gray scale intensity of the neutron radiographs is used to quantify the water uptake rate. The sudden decrease in the gray scale intensity indicates the time when the water front reached the region of interest being analyzed along the fracture. Figure 2 shows the water front height vs square root of time for 0.1 mM and 0.9 mM ADS/OTAC 1:1 mixtures. The water front propagation was plotted against the square root of time and the slope indicates the sorptivity or the water uptake rate in the fracture for each surfactant solution concentration. From Figure 2 sorptivity was measured to be 119 and 50.2 mm/sec1/2 for 0.1 mM and 0.9 mM ADS/OTAC 1:1 mixtures, respectively, indicating a reduction of 58% in sorptivity of shale fracture when the mixed surfactant concentration was increased from 0.1 mM to 0.9 mM.
Figure 3 shows the comparison of sorptivity for both pre-exposed and unexposed shale fractures at different surfactant loading conditions. The sorptivity for pre-exposed shale fractures was significantly reduced compared to that of the unexposed shale fractures for all surfactant concentrations (Figure 3(a)). The difference in sorptivity is larger (96% reduction for pre-exposed shale fracture at 0.1 mM ADS/OTAC 1:1 mixture) at low surfactant concentrations (less than 0.3 mM). During water imbibition into unexposed shale fractures, such as will occur during and directly after hydraulic stimulations, no wettability effect was observed on capillary pressure due to lack of surfactant adsorption at the fracture interface. The surface tension also remains unaltered compared to pure water at low surfactant concentration (less than 0.3 mM). Consequently, due to the unaltered surface wettability and surface tension, the sorptivity for unexposed shale fractures remains similar to pure water at surfactant concentrations up to 0.3 mM. Due to drastic reduction in capillary pressure (a wettability effect) for pre-exposed shale fractures, the difference in sorptivity is larger between unexposed and pre-exposed shale fractures at surfactant concentration up to 0.3 mM. This result indicates how the longer contact times associated with the well shut-in period may be a very important step for improving gas recovery due to enhanced gas relative permeability in water-filled fractures.