Reports: ND156230-ND1: Hypervalent Iodine-mediated Transformation of Silyl-substituted Hydrocarbons
Annaliese K. Franz, PhD, University of California, Davis
This annual report presents the ongoing work to discover a method to transform a carbon-silicon bond into a carbon-nitrogen bond to access chiral amines in an aza-variant of the Tamao-Fleming reaction, which would provide complementary method to functionalize silyl-containing molecules. To accomplish this goal, our initial work focused on utilizing silylamines with direct activation for alkyl migration with a focus on hypervalent iodine(III) reagents. Consistent issues occurred with the synthesis of silylamine compounds, either isolated or formed in situ. Using several strategies, we tested a variety of nitrogen reagents, but reliable conditions for the successful transformation to an amine has not yet been developed. While our strategy utilizing an oxidation with hypervalent iodide is promising, current methods using these reagents did not afford the amine product.
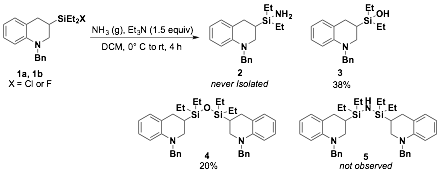
Addition of ammonia to silylchloride reagents to obtain silylamine substrates
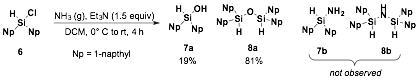
Our initial results suggested that silylamine 2 was being formed from addition to 1a as the major product in a mixture; However, inconsistent reaction results led us to perform further structure determination and we determined that formation of silanol 3 and disiloxane 4 are the major species observed. Knowing that silylchlorides are also sensitive to hydrolysis, the silylfluoride 1b was reacted in the presence of ammonia, but also only yielded silanol 3 as determined by 1H NMR spectroscopy and ESI-MS. When we tested the addition of ammonia to binaphthylsilylchloride 6 we only isolated disiloxane 8a as the major product with silanol 7a observed as the minor product (Scheme 1). The inability to access the silylamine (2 or 7b) is attributed to the weakness of the silicon-nitrogen bond, which upon exposure to air, water or silica gel can be rapidly hydrolyzed to the silanol.
Scheme 1. Addition of ammonia to silylchlorides yield silanols and disiloxanes instead of desired silylamine
Hydrosilylation of imines for transformation of silicon-nitrogen bonds to carbon-nitrogen bonds by hypervalent iodide
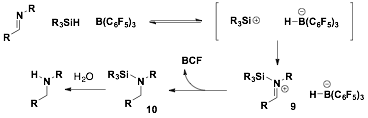
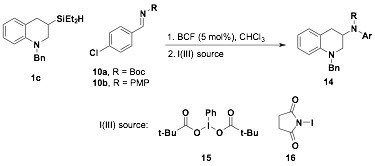
Based on the mechanistic rationale for hydrosilylation of imines to form in-situ silyl-amines (e.g. 10, Figure 1) we investigated this route with the plan to add oxidants to cause the rearrangement to yield the desired amines. We first began investigating N-Boc aldimines 11 with tetrahydroquinoline 1c using BCF catalyst. Allowing the reaction to proceed and followed by either aqueous or silica plug work up, the free amine 13 was isolated in 52% yield (Eq. 1). This demonstrated that silane 1c can interact with BCF and hydrosilylation could occur with this substrate, but even if silylamine 12 could be forming in the reaction, it was not isolated.
Figure 1. Mechanism of Hydrosilylation of Imines with BCF as an alternative route to access silylamines
|
|
Understanding that the silylamine 12 could not be isolated, we sought to generate the silylamine in-situ followed by addition hypervalent iodide species that could cause the rearrangement to form the carbon-nitrogen bond to access amine 14. Aldimines 10a,b was complexed with catalytic amounts of BCF followed by addition of silane 9. However, no additional product formation was observed by TLC. When the hypervalent idodide species (e.g. 15 or 16) was added to the reaction the reaction solution turned green within 1 h (Figure 2). Based on TLC, the N-iodosuccinimide (16) consumed the silane but ESI-MS and 1H NMR did not indicate product formation. The I(III) source 15 was less reactive then 16 and again nothing could be identified. We hypothesized that the in-situ conversion of the silylamine to amine 14 was unsuccessful because low conversion to the silylamine was observed and the resulting nitrogen-silicon bond was too labile. Additionally, it appears that once the iodide species were added to the reaction mixture, the iodide assisted in the cleavage of the nitrogen silicon bond instead of the desired migration.
Figure 2. Hydrosilylation of imines followed by I(III) oxidation
Rhodium-catalyzed selective C-H insertion into tetrahydroquinolines over silanes
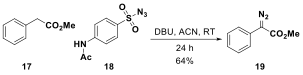
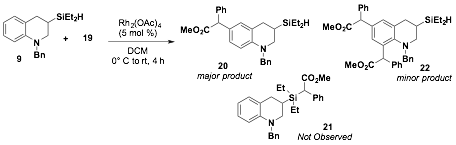
In parallel to investigating a silicon-nitrogen bond forming reaction in the aza-Tamao-Fleming reaction, we also investigated a method to access silicon-carbon bonds by inserting a reactive diazo species into the Si-X bond of a silane or silylfluoride. Additionally, we envisioned that under the right conditions the newly formed carbon-silicon bond could be rearranged to form a new carbon-carbon bond. The use of donor-acceptor diazo compounds has become an increasingly important area of research for C-H activation as well as for insertion of Si-H bonds. A model donor-acceptor diazo compound 19 was prepared from benzylacetate 17 and a diazo-transfer reagent 18 in moderate yields (64%). Diazo 19 was then complexed with Rh2(OAc)4 and silyl-containing tetrahydroquinoline 9; however, insertion into the Si-H bond was not observed. What was observed was insertion of benzylacetate into the aromatic ring para to the amino directing group, forming 20 in modest yields (47%) (Scheme 2). Furthermore, a second addition of benzylacetate into the aromatic ring was also identified as a minor product 22 of the reaction by ESI-MS and 1H NMR spectroscopy. Testing an unsilylated tetrahydroquinoline in the diazo addition reaction with Rh2(OAc)4, resulted in products with similar substitution patterns, suggesting that the reactivity is not to be into the presence of the silyl group.
Scheme 2. Rh2(OAc)4 catalyzed diazo additions to tetrahydroquinolines
Currently, the benefits and goals remain the same but our methodology involves a new strategy. Based on our preliminary results which have yielded no desired transformation, we have now designed a new strategy for transformation that will hypothesize will address the challenges identified. We still have plans to investigate alternative mechanistic approaches with azides and Rh-nitrene reagents.
Advancing Scientific Education and Student training
To date, this project has contributed to the scientific education and laboratory training of two graduate students who have been trained in synthesis, methodology development, and chemical analysis. An additional graduate student also contributed to the development and preliminary results for the project, but was not funded by the grant. In the upcoming year, the combination of synthesis and mechanistic investigations will provide excellent training for one new graduate student (TBD) and an undergraduate student (TBD) who will begin to work on the next phase of the research goals.