Reports: DNI357058-DNI3: Non-Innocent Heterobimetallic Scaffolds for Carbon Dioxide Reduction
Caroline T. Saouma, PhD, University of Utah
PREVIOUS RESULTS
Starting from a tris(phosphine) ligand scaffold, we are tethering on chemical moieties to perturb the second coordination sphere. The proposed complexes are shown in Figure 1. To probe the effect of Lewis-acids, a scaffold that features a second metal binding site is proposed. To probe the effect of Brønsted-acids, a scaffold that features an appended amino functionality is proposed.
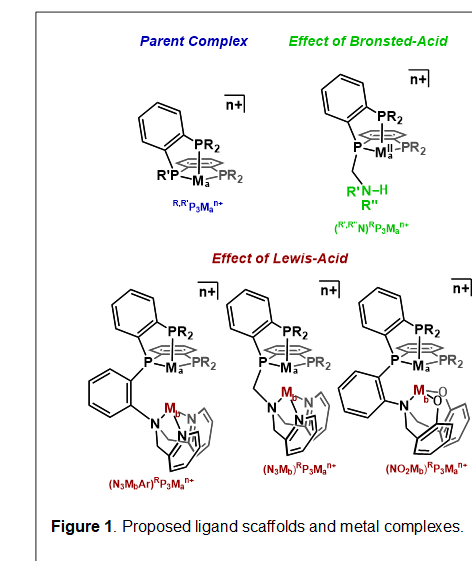
Currently, many of the proposed ligands have been prepared. the N3RP3 scaffold has been prepared, with R = Ph and iPr (Figure 1, red middle). These novel ligands can be made on multi-gram scale in five steps from commercially available chemicals. The syntheses of the R’,R”NRP3 ligands (Figure 1, green) has also been achieved, with R = Ph or iPr and R’ = R” = Bz or Ph). The control ligands, R,MeP3 (R = Ph, iPr) in which there is no tethered site for attachment of an acid have also been synthesized (Figure 1, blue). The final two ligand types, shown in the bottom of Scheme 1, will be targeted in the upcoming year. We anticipate that these have heightened stability relative to the current N3RP3 ligands (vide infra).
Treatment of N3iPrP3 with a variety of metal salts give corresponding complexes, as shown in Figure 2. Worth noting, treatment with FeCl2 gives a fluxional paramagnetic species which coordinates the iron via the three N-ligands (confirmed by XRD, and 31P NMR shows a broad resonance for the phosphines). Upon removal of a chloride withTlPF6, a color change ensues and now the 31P NMR resonance is absent, suggesting that the iron migrates to the P3-binding site. While the di-iron structure does not suggest any communication amongst the two metals, the di-ruthenium structure clearly indicates that the ligand can support structures whereby the two metals communicate.
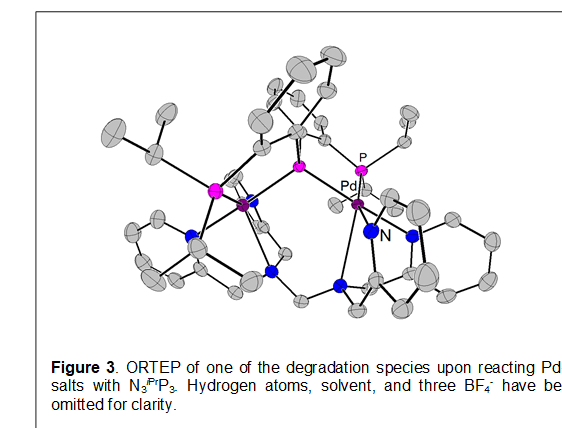
Attempts to metallate group 10 metals with N3iPrP3 have all been unsuccessful. Reaction of the ligand with Pd(II) salts invariably leads to ligand degradation and formation of what is thought to be the phosphide ligated species, iPrP3Pd(MeCN)+ (identified by NMR analysis) along with the 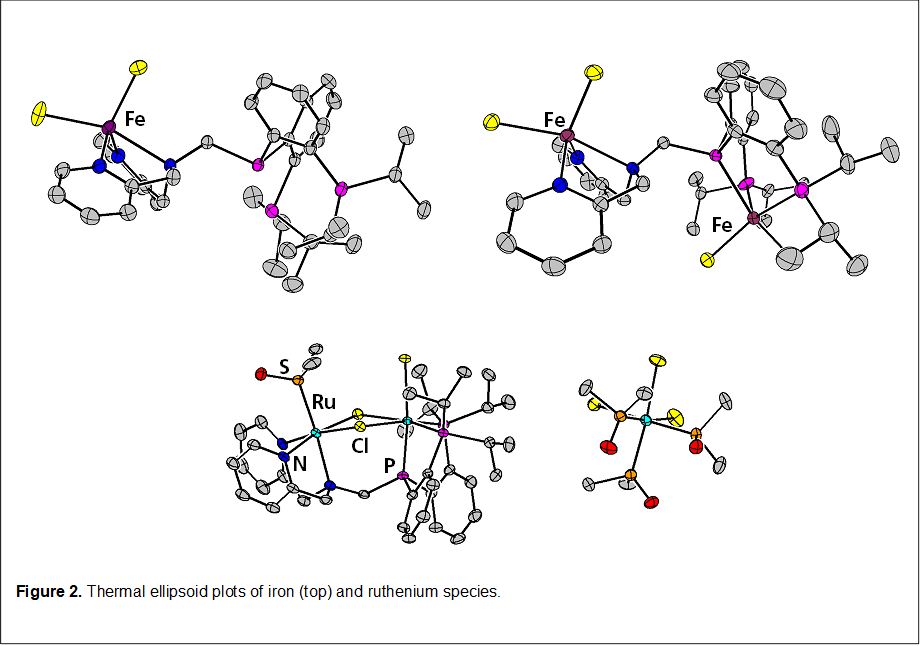 dimeric trianionic phosphide species shown in Figure 3. Similar results are obtained with Ni salts. Thus, an unusual C-N coupling occurs, with loss of a CH2 functionality (as the reaction is not clean, we are not speculating as to where this has ended up).
dimeric trianionic phosphide species shown in Figure 3. Similar results are obtained with Ni salts. Thus, an unusual C-N coupling occurs, with loss of a CH2 functionality (as the reaction is not clean, we are not speculating as to where this has ended up).
These results have prompted us to go back to our ligand design, and commence syntheses of the other two ligands shown in the bottom of Figure 1.
The palladium complex iPr,MeP3Pd(MeCN)22+ has been investigated for electrocatalytic reduction of CO2 in the presence of HBF4. This “control” complex was chosen for initial studies for its similarity to the Dubois catalysts. We found that this complex does function as a CO2 reduction catalyst to give CO selectively. Foot-of-the-wave analysis is ongoing, as are studies aimed at characterizing intermediates. We anticipate a manuscript on this project being prepared within the next year.
The Fe complexes have also been studied via electrochemistry, but neither of them serve as catalysts for CO2 reduction.
Very recently, Fe and Co complexes of R’,R”NRP3 have been prepared. Again, attempts to prepare Pd or Ni species with this ligand resulted in cleavage of a P-C bond and formation of a phosphide, suggesting an inherent instability of the P-CH2-N functionality on group 10 metals. Though the electrochemistry of the Fe and Co species is yet to be explored, these complexes do serve as hydrogenation catalysts, converting CO2 to MeOH under elevated pressures. These results are promising.
FUTURE WORK
Over year 1 of funding, we were able to prepare a variety of complexes, which uncovered the design flaw of the P-CH2-N linkage not being stable with Pd. This has forced us to re-design our proposed ligands. Over the next year of funding, the following work will be done:
Completion of electrocatalytic studies using the Pd species described above. As the Pd complex is charged, studies will be done with excess group I salts, to see if there is an effect of Lewis acid. We will also explore the electrocatalytic reduction of CO2 in the presence of various amines. This will generate carbamate and carbamic acid in solution, which may serve as CO2 surrogates. This has the benefit of concentrating the CO2 in solution, and may also provide a substrate that is more readily reduced.
Preparation of related ligands that feature a more robust P-aryl-N linkage, and studies of metal complexes that feature this new ligand scaffold. Of interest will be the ligand that features an amine-bisphenolate, as this will lower the positive charge on the metal and increase the likelihood of binding a Lewis acid.











