Reports: DNI156603-DNI1: Novel Nitrogen-Substituted (lamba)3-Iodanes for the Functionalization of Chemical Feedstocks
Sarah Wengryniuk, PhD, Temple University
Research Period Summary:
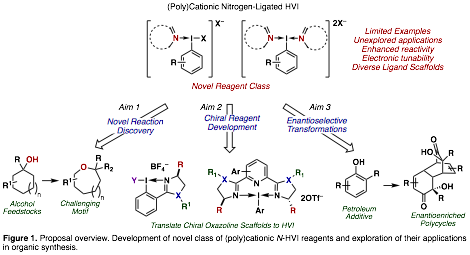
This DNI award aimed to advance (poly)cationic hypervalent iodine reagents, heterocyclic nitrogen-ligands (N-HVI) (Figure 1), as a novel and powerful class of synthetic reagents. This overarching goal was approached through three specific aims involving both focused reagent development as well as synthetic derivatizations of chemical feedstocks, and excellent progress has been made towards all of these goals during the 2016-2017 research period.
– Aim 1 focused on the further development of a novel electrophilic alcohol ring expansion reaction for the synthesis of challenging medium-sized ring ether scaffolds. To date, this methodology has been published for the rearrangement of tertiary, benzylic alcohols to access diverse benzoxepanes as well as expanded to aliphatic secondary alcohols. Through this effort our existing library of N-HVI reagents has expanded, setting the stage for further characterization.
– Aim 2 focused on the development of chiral bisoxazoline hypervalent iodine reagents in three classes. An efficient synthesis of these reagents has been developed and a tBu derivative has been successfully synthesized and characterized by x-ray crystallography
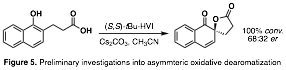
– Aim 3 looked to apply the chiral bisoxazoline N-HVI to the asymmetric functionalization of petroleum feedstocks. Preliminary investigations into asymmetric phenol dearomatization have given promising results, laying groundwork for further development.
Research Outcomes: The progress outlined in the following report has led to several tangible research outcomes:
– Federal Funding: Results obtained during the first year of DNI-56603 were crucial preliminary findings that were included in a NIH R01 proposal which was successfully funded (R01-GM123098) in April of this year.
– Publications: The work outlined in Aim 1 resulted in a publication in Organic Letters, however this publication was accepted prior to funding of this proposal. Subsequent efforts towards utilizing secondary alcohols has led to a manuscript that is currently under preparation with planned submission to Nature Chemistry. Additionally, our investigations into fundamental N-HVI reactivity (Aim 1, 2) led to interest in their potential as oxidants in transition metal chemistry. In conjunction with this work, we published a review, Hypervalent Iodine Reagents in High-Valent Transition Metal Chemistry (Sousa, F. C. S.; Tierno, A. F.; Wengryniuk, S. E.*, Molecules, 2017, 22, 780.) in which the PRF was acknowledged.
– Presentations: The work has been presented at numerous national and regional meetings by both the PI, graduate students, and postdoctoral fellows. This includes national and regional ACS meetings, Gordon Research Conferences, local undergraduate poster sessions, and internal Temple Research Symposia.
– Student Support & Training: The research funding provided student support for one female graduate student (Walters, Jennifer) as well as support for one incoming first year student (Accocella, Ryan) to work in the Wengryniuk laboratory. In addition to those directly supported, numerous undergraduates as well as additional postdoctoral researchers collaborated on this work. A female undergraduate was awarded a prestigious Frances Velay Fellowship award to support her summer research because of her efforts on developing the electrophilic alcohol rearrangements.
Research Progress
Electrophilic Alcohol Ring Expansions
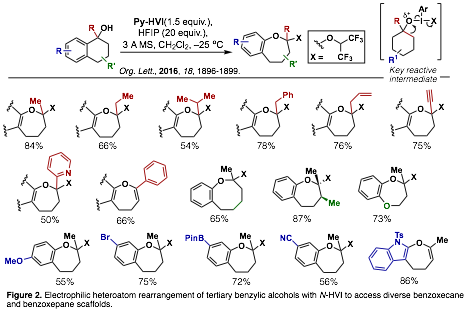
Medium-ring ether scaffolds are ubiquitous functional motifs in bioactive molecules, however efficient methods for their synthesis remain limited. We proposed the development of a novel approach to these scaffolds through the electrophilic heteroatom rearrangement of simple alcohol feedstocks. This transformation was found to be uniquely enabled by N-HVI reagents, providing insights into their unique reactivity and synthetic potential. To date, we have developed an efficient synthesis of diverse benzoxepane and benzoxecane scaffolds through the rearrangement of benzylic tertiary alcohols (Org. Lett., 2016, 18, 1896-1899, Figure 2).
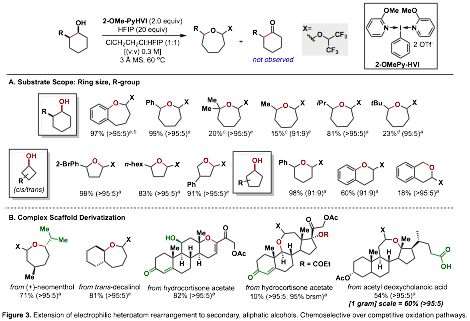
Building on these results, we have expanded this methodology to the rearrangement of secondary aliphatic alcohols, a much more challenging transformation due to the possibility for competing a-elimination pathways (Figure 3). Extensive optimization efforts led to the finding that C2-cis-substituted secondary alcohols, upon treatment with [2-OMe-Py]N-HVI in DCE:HFIP at 60 ¼C led to highly selective rearrangement over competing oxidation pathways. A manuscript is in preparation for submission to Nature Chemistry outlining this work.
Chiral Bisoxazoline N-HVI Reagents
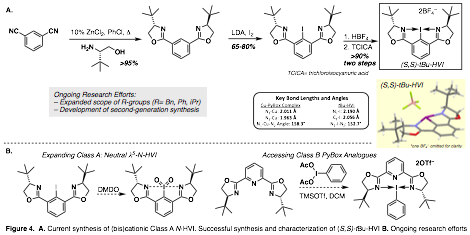
Chiral hypervalent iodine reagents have the potential to be highly versatile and impactful synthetic reagents. Unfortunately, progress in this area has been hindered by a limited pool of chiral scaffolds and low enantioselectivities. We proposed the development of a novel class of chiral N-HVI reagents based on the ubiquitous bisoxazoline scaffold. To date, we have successfully developed an efficient synthetic route to the Class A chiral N-HVI scaffold (Figure 4A) and have synthesized (S,S)-tBu-derivative which was also characterized by x-ray crystallography (Figure 4A, inset). As hypothesized the structural parameters of (S,S)-tBu-HVI are analogous to those seen in Cu-PyBox complexes, providing indications that this system could provide high levels of selectivity. Current efforts are focused on expanding the library of these Class A reagents (R-groups), accessing neutral l5-analogues of Class A as well as accessing Class B PyBox-based scaffolds (Figure 4B).
Initial investigations into the reactivity of these novel reagents have been conducted in the context of asymmetric dearomatization. Model substrate XX was selected due to its use in numerous reports on chiral HVI-mediated dearomatizations. To date, it has been found that XX can successfully mediate dearomatization with moderate levels of selectivity (Scheme 3). Continued efforts to improve the selectivity including reagent scaffold optimization, temperature, and additives as well as preliminary efforts to explore other transformations are underway.
N-HVI Library Synthesis
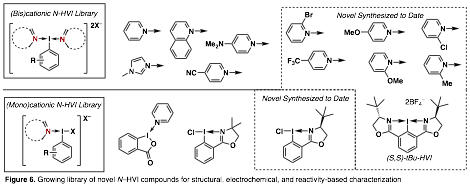
At the outset of our research, there were extremely limited reports on N-HVI reagents and only 7 were known. To date we have expanded this library, with a focus on steric and electronic diversification and this effort is ongoing (Figure 6). In order to systematically characterize the effect of these parameters, we have established robust cyclic voltammetry conditions and efforts to begin characterizations are underway. The goal is to establish trends based on key reagent parameters and to use this data as predictive tools for reactivity and these efforts are a key focus of the upcoming research period.