Reports: UR1055521-UR10: Electrochemical Interactions between Conjugated Polymers and Polymerizable Salts
Janelle M. Leger, PhD, Western WA University
Annual Report: ACS PRF UR: Electrochemical Interactions between Conjugated Polymers and Polymerizable Salts, Janelle Leger, Sept. 1 2016 - August 31 2017.
Goals of the Project:
Several emerging organic electronic technologies, known as 'iontronic' devices, utilize the unique mixed ionic/electronic conducting character of conjugated polymeric materials. In the majority of iontronic devices, all steps of device operation are reversible; once the bias that assembles the ion profile is removed, the device discharges and the ions redistribute. In our lab, we have developed a process by which a remarkably robust fixed junction can be formed in commercially available conjugated polymers that is not subject to ion relaxation or redistribution. We achieve this by replacing the salt used in traditional iontronic devices with polymerizable ionic liquids (PILs) that can potentially be polymerized via radical initiation. While the effects of including PILs as the source of counterions on device characteristics are well understood, little is understood about the mechanism that leads to these effects. Our working hypothesis is that a covalent bond forms between the PIL and the conjugated polymer, initiated by the formation of a radical during the electrochemical doping process of the conjugated polymer itself. However, no direct evidence for covalent bond formation has been shown. The overarching goal of this work is to elucidate the nature of the electrochemical interactions between these species and the resulting structures formed, determine whether the reaction results in a covalent bond between the PIL and the CP, and determine how the reaction and any resulting structural changes affects the physical properties of the CP. We also plan to explore the design of new PILs with improved properties for a variety of applications.
Research Progress:
In our last annual report, I described some interesting results in which we found that the molecular weight of our conjugated polymer, MEH-PPV was decreasing upon oxidation using the polymerizable ionic liquid ATOAAS. In our attempt to follow this line of research, we discovered eventually that our ATOAAS was not pure. Specifically, it was contaminated with inorganic impurities which cannot be detected by NMR. Purity of the PIL with respect to inorganic ions is extremely important and quite difficult to assess and control. During summer 2017 most of my student's time (Ariel Garcia) was spent rectifying this problem through tweaks to our synthetic procedure, modifications to our equipment (Schlenk line and vacuum pump), characterization procedures, purification of our starting materials, and cleaning procedures. As a result, we believe that we overcame this problem toward the end of this past summer, and are currently able to successfully reproduce ATOAAS that is as pure as we are able to assess. Unfortunately, because most of the work of this grant depends on the availability and purity of this material, our overall progress over the past year has been slow as we worked to understand the nature of the problem and worked to solve it. Nevertheless, we have made some important progress that will allow us to take full advantage of the next year of funding, beyond perfecting the ATOAAS synthesis, as described below.
Air-free
electrochemistry: We built an
apparatus for air-free electrochemistry, specifically cyclic voltammetry. This
setup allows us to perform every aspect of the setup in an inert atmosphere
(glove box), from the polymer deposition to the preparation of the electrolyte.
The cyclic voltammograms prepared using this
technique are particularly clean and reproducible (Figure 1). In addition, as
we progress to more advanced e-chem techniques, this
setup will allow us to eliminate side-reactions caused by oxygen.

Figure 1: Air-free CV setup
Rotating disk electrode: We were able to purchase and install a rotating disk electrode which will allow us to move forward on the proposed advanced electrochemical characterization and provide us important information on diffusion coefficients.
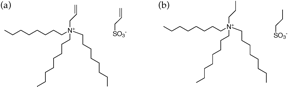
Synthesis of the PTOAPS: We began work on the saturated ATOAAS equivalent, PTOAPS. We have accomplished all steps of the reaction, but our product is not pure by NMR. Therefore, we are currently working on optimizing this synthesis.
Figure 2: Molecular structure of (a) ATOAAS and (b) the equivalent structure, lacking the characteristic double bonds (PTOAPS).
Overall outcomes:
During this funding period our progress has been slow due to a significant roadblock in the synthesis of our workhorse PIL, ATOAAS. As a result of this setback, we did not spend one of the two undergraduate stipends that we had allotted this summer, and plan to use three in order to complete the proposed work next summer. There have also therefore been no publications during this funding period. There have, however, been important outcomes in terms of student preparation. Two undergraduate students (James Bauman and Ariel Garcia) and one M.S. student (Sarah Clark), were given the opportunity to carry out part time research during the 2016/17 academic year. Ariel carried out full time research during Summer 2017 as well. With the ATOAAS synthesis bottleneck solved, Ariel and Sarah will continue with their research during the 2016/17 academic year. In addition, I have taken on two more undergraduate students this year on this project (Marcus Brittain and Kyle Young).
Plans for the next funding period:
Over the next funding period we expect to be productive, with usable materials and three undergraduate research assistants. Our goals are still in line with those of the original proposal, including electrochemical characterization of MEH-PPV with ATOAAS and the resulting optical and electronic characterization, synthesis of PTOAPS, and synthesis of novel PILs.