Reports: DNI756387-DNI7: A Versatile Catalytic Approach to Polyether Synthesis
Nathaniel Alexander Lynd, PhD, The University of Texas, Austin
Impact of the Research
Polyethers represent a major class of industrially-produced materials that are used in all aspects of industry from automotive applications to personal care. The synthesis of polyethers derived from epoxides has developed form the earliest days of polymer science.[1] However, there is currently no widely-used consensus technique for general-use polymerization of epoxides to controlled molecular weight beyond 1000~5000 g/mol in spite of the versatility that polyethers encompass to address new technological challenges in the 21st century.
As our new catalyst design ansatz, we explored the structure and mechanism of the Vandenberg aluminum acetylacetonate chelate catalyst that was reported in the 1950s.[2] The Vandenberg catalyst possessed many of the requisite properties that are desirable in a modern polymerization methodology. However, the statistical nature of the catalyst synthesis prevented the establishment of mechanistically informative structure-kinetic relationships, and no further improvements in catalyst performance in the intervening decades since the original discovery.
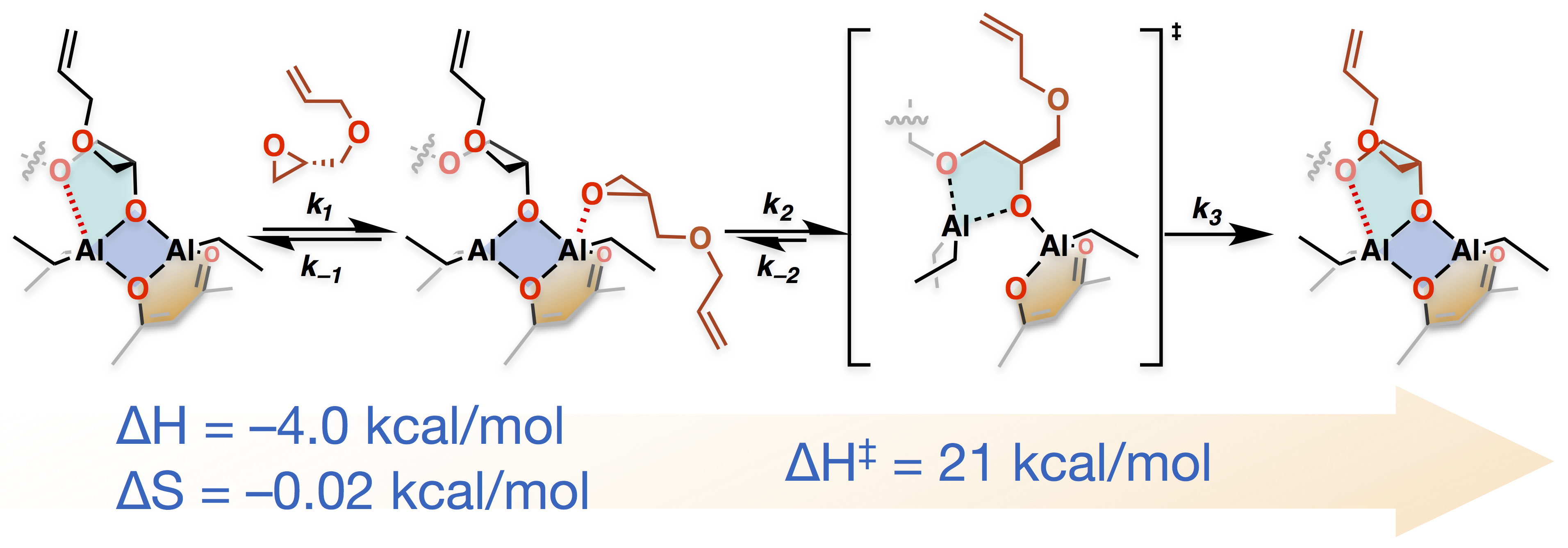
Figure 1. The structure and mechanism of the Vandenberg catalyst instigated our research into catalytic approach to polyether synthesis. Shown above is our proposed structure and mechanism for the classical Vandenberg catalyst. The transition state structure is proposed to be a mono(µ-oxo)-dialuminum and accounts for the high activity of the catalyst.
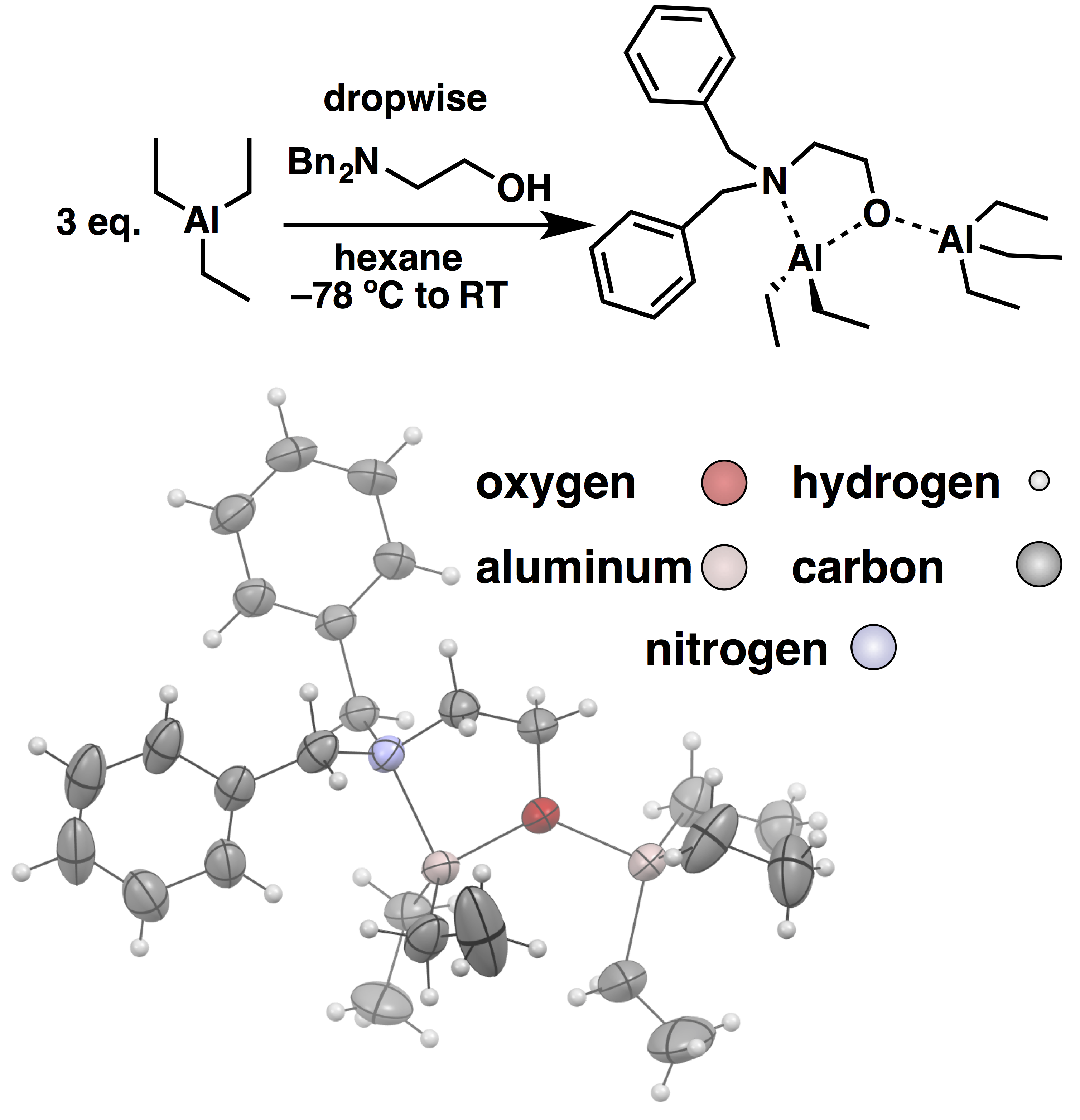
Using modern tools of characterization, we analyzed the structure, kinetics, and mechanism of the Vandenberg catalyst. Significantly, we observed polymerization behavior that was consistent with livingness (no self-termination), and a single species as being responsible for monomer enchainment. Based on these experimental results, we proposed that the catalyst resting state structure was represented by a rigid bis(µ-oxo)-dialuminum ring, and the transition state for monomer enchainment proceeded through an open mono(µ-oxo)-dialuminum (see Figure 1). In the course of this fundamental investigation, we synthesized a series of bis(µ-oxo)-dialuminum species that produced the same stereochemistry in the resultant polyether that the original Vandenberg catalyst did. However, the activity toward epoxide polymerization of the new bis(µ-oxo)-dialuminum (BOD) species was low. While the BODs were mechanistically informative, we had to go back to the proverbial drawing board in order to make progress on a general-used technique for epoxide polymerization. On a Friday afternoon, Christina Rodriguez was attempting a final BOD synthesis utilizing a N,N-dibenzyl-aminoethanol. An off-stoichiometric product crystallized that polymerized neat epichlorohydrin to 10,000 g/mol in a matter of minutes! Our attention shifted to the structure and mechanism of the new initiator. Significantly, X-ray crystallography revealed that the structure of the new initiator was a mono(µ-oxo)-dialuminum (MOD) that was similar in structure to the transition state of the Vandenberg catalyst as shown in Figure 2.
Figure 2. The first mono(µ-oxo)-dialuminum (MOD) initiator was synthesized from N,N-dibenzylaminoethanol and triethylaluminum. The initiator exhibited high activity for epichlorohydrin polymerization. Shown above is the structure obtained by X-ray crystallography.
The behavior of the initiator was explored using a standard battery of tests used to explore polymerization behavior. Significantly, the MOD-initiated polymerization of epoxides was consistent with a controlled living polymerization. Molecular weight was controlled by the polymerization stoichiometry, and chain-extension experiments demonstrated living behavior.[3] The first-reported MOD initiator was used to demonstrate versatility in monomer substrates and also polymer architecture.
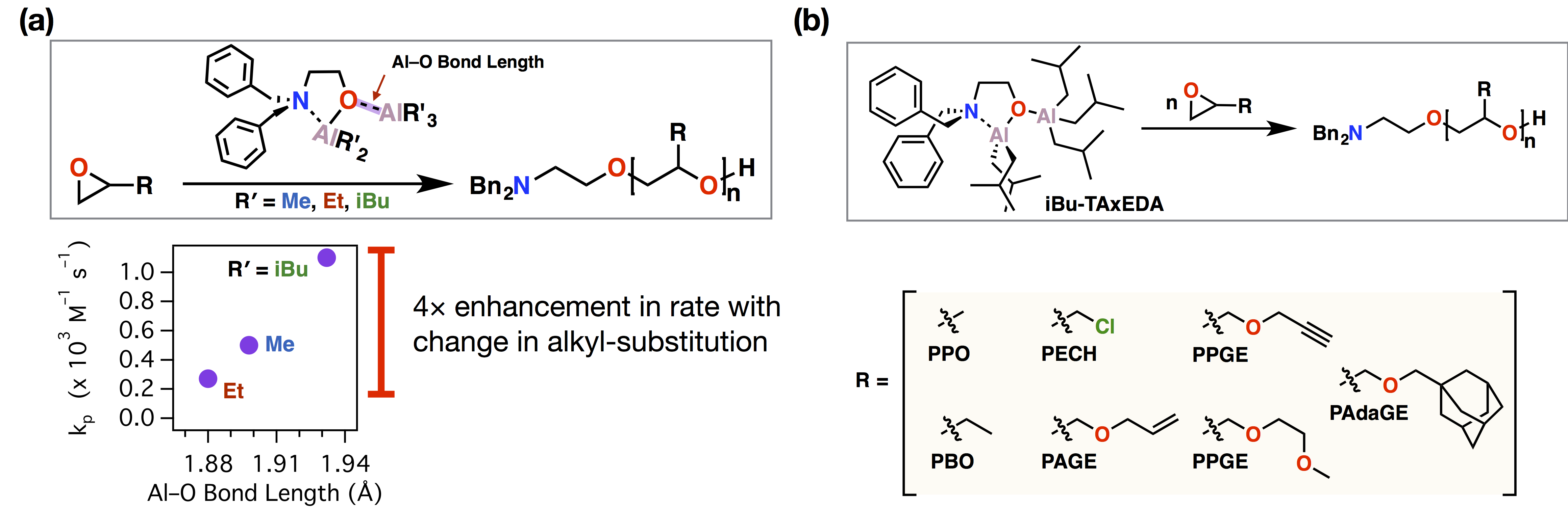
The next stage of our study focused on systematic variation in MOD composition by varying the alkyl substitution on the aluminum alkyl groups. The results of the study were surprising, and proved to be mechanistically penetrating. The bond length between the datively-bound trialkylaluminum and the aluminum alkoxide on the initiator proved to correlate to the rate of polymerization in an analogous manner to traditional metal alkoxides. The bond in question, and the change in propagation rate constant (kp) as a function of the Al-O dative bond length is shown in Figure 3a. The increased rate and selectivity in polymerization provided access to new materials that were unavailable to functional polyether materials. An expanded scope of polymer structures synthesized with the most active mono(µ-oxo)-dialuminum published to date is shown in Figure 3b.[4]
The first year of the ACS-PRF Doctoral New Investigator supported research has produced fundamental mechanistic insight for the classical Vandenberg catalyst, and the new mono(µ-oxo)-dialuminum initiators that represent an excellent platform for general-use epoxide polymerization. We are excited about the possibilities for both new fundamental discovery, and practical application of this chemistry in the years ahead.
Figure 3. (a) Variation in MOD structure led to a mechanistically-informative four-fold enhancement in epoxide polymerization rate. (b) The increased selectivity and rate led to the polymerization of new monomers to higher molecular weights than were previously obtainable.
Impact of ACS-PRF-DNI support on my Career
The ACS-PRF DNI grant was the first single-PI grant that was awarded to my new academic research group. The support was instrumental in launching the successful efforts of my group in polymerization catalysis for epoxides. This next year my students and I will continue to develop this system for greater utility, gain new mechanistic insight, and to publish those findings in peer-reviewed literature and present them at national ACS meetings.
References
1. Staudinger, H. and Lohmann, H. ҆ber Hochpolymere Verbindungen. 81. Mitteilung. †ber Eukolloides PolyŠthylenoxyd" Justus Liebigs Annalen der Chemie 505, no. 1 (1933): 41-51.
2. Vandenberg, E. J. "Catalysis: A Key to Advances in Applied Polymer Science" ACS Symposium Series 496, (1992): 2-23.
3. Rodriguez, C. G., Ferrier, R. C., Jr, Helenic, A., and Lynd, N. A. "Ring-Opening Polymerization of Epoxides: Facile Pathway to Functional Polyethers via a Versatile Organoaluminum Initiator" Macromolecules 50, no. 8 (2017): 3121-3130.
4. Ferrier, R. C., Imbrogno, J., Rodriguez, C. G., Chwatko, M., Meyer, P. W., and Lynd, N. A. "Four-Fold Increase in Epoxide Polymerization Rate with Change of Alkyl-Substitution on Mono-_-Oxo-Dialuminum Initiators" Polymer Chemistry 8, no. 31 (2017): 4503-4511.