Reports: DNI454860-DNI4: High-Resolution EPR Characterization of the Ligand Structure in Vanadium Porphyrin Complexes
Stefan Stoll, PhD, University of Washington
The goal of this project is to build a better understanding of the magnetic structure of vanadyl porphyrins using high-resolution Electron Paramagnetic Resonance (EPR) spectroscopy techniques. This will provide a new approach for the speciation of vanadyl porphyrin compounds in crude oil, a prerequisite for improving methods of managing vanadyl porphyrins, which is an ongoing problem in oil refining. High-resolution EPR spectroscopy resolves variations in the structure of various porphyrin ligands via their magnetic signature.
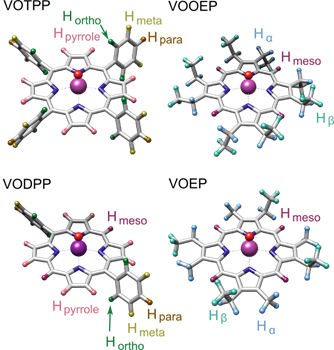
In this project, we examined a series of model vanadyl compounds in order to develop and assess the resolving power of this approach (see Fig 1). The major difference in the magnetic structure among these vanadyl porphyrins is in the sidechains attached to the porphyrin macrocycle frame.
Figure 1: Structures of model vanadyl porphyrins. Vanadium is purple, oxygen is red, nitrogens are blue, carbons are gray, and the protons are color coded and labeled in groups according to their structural locations.
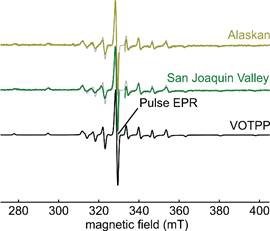
The magnetic structure of vanadyl porphyrins is dominated by the paramagnetic vanadyl ion. A representative example (VOTPP) of a CW vanadyl porphyrin spectrum along with the spectra of two different crude oils are shown in Fig. 2. The lack of resolution in the CW EPR spectra prevents speciating the porphyrin ligands in the crude oil samples.
Figure 2: CW EPR spectra of pure VOTPP and crude oil samples from Alaskan and San Joaquin Valley sources. A gray overlay of the VOTPP spectrum demonstrates the similarity of the spectra, the gap in the crude oil spectra is due to the removal of the organic radical signal found in crude oil. The spectral position used for pulse EPR is indicated.
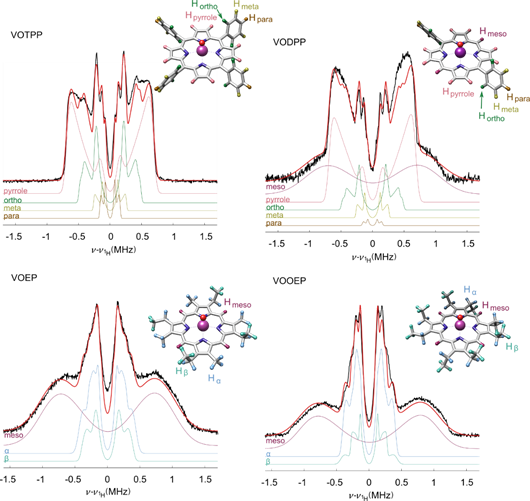
To achieve this speciation, we used X-band Mims Electron Nuclear Double Resonance (ENDOR), a pulse EPR technique. This resolves the magnetic hyperfine interaction between the unpaired 3d electron on V(IV) and the ligand protons. The ENDOR spectra are shown in Fig. 3. It is evident that the spectra differ strongly among the various porphyrins. This demonstrates that ENDOR can be used as a technique for ligand speciation.
Figure 3: 1H ENDOR spectra of model vanadyl porphyrins. The horizontal axis shows the offset form the 1H Larmor frequency. Black: experimental data, red: simulation, remaining colors: simulations of spectral components due to individual protons. The ligand protons of the inset structures are color coded as their corresponding spectral components.
We assigned spectral features to chemically distinct protons by a combination of spectral simulations and quantum chemical calculations. These assignments are shown in Fig. 3. Both VOTPP and VODPP have pyrrolic, ortho, meta and para protons in common and the spectral simulations were made with equivalent parameters. The spectra of VOEP, and VOOEP have features due to the methyl and ethyl ligands in these porphyrins. The peak positions for these methyl and ethyl protons are equivalent as well. VOEP, VOOEP, and VODPP have a broad meso proton feature. Importantly, we are able to analyze the spectral intensity for each chemically distinct group of protons and extract the number of the protons in that group. Therefore, ENDOR spectra allow proton counting and chemical speciation of the porphyrin ligand.
Figure 4: Comparison of VOOEP/VOEP mixtures measured with Mims ENDOR vs. the solution as prepared. The vertical gray bars are the error in ENDOR fit and the horizontal gray bars are the error introduced in mixing. The red dashed line is the regression fit, with the equation and RMSD value relative to the model (solid gray line).
We prepared mixtures of VOOEP and VOEP to further test the speciation capability of this approach. As shown in Fig. 4, the mixture composition can be quantified reasonably well using the ENDOR approach. This is noteworthy given that the difference between the two porphyrin compounds is in the number of methyl or ethyl groups (4 methyl + 4 ethyl in VOEP vs. 8 ethyl in VOOEP).
In summary, our results show that ENDOR spectra are sensitive to the number and type of protons in porphyrins and therefore allow ligand speciation in a mixture of very similar vanadyl porphyrins. This method is useful for examining crude oil samples from various sources to better understand the speciation of naturally occurring porphyrins.