Reports: ND354820-ND3: Reductive Elimination at Group 14 Elements: A Pathway Toward Catalysis
Rory Waterman, University of Vermont
I. Research progress.
The dour news, but for god reason, is that research progress was somewhat limited in this final year. The department moved from the 1960s era Cook Physical Sciences Building to the newly completed Discovery Hall. While all efforts were made to minimize disruption, the complete shutdown of my lab and department, move, and restart operations cut at least a quart of working time out of the year. Overall, research progress was far less substantial in the two critical areas of the work than I would have hoped. We continued on the stated state goal of observing facile reductive elimination from tin(IV) centers, but the progress in tin(IV) sigma bond metathesis has been an intriguing area that has been the most fruitful in this period.
II.a. Sigma bond metathesis
We had identified that tin(IV) derivatives Cp*2SnCl2 and Ph2SnCl2 as pre-catalysts for phosphine dehydrocoupling and hydrophosphination. In the course of those studies, we accrued some evidence that may support the notion that these tin(IV) compounds are engaging in sigma-bond metathesis. The evidence to suggest this conclusion arises from a competition experiment that provides information about the relative rates of E–H versus E–D bond activation. In that work, hydrophosphination of styrene was performed using an excess of an equivalent mixture of diphenylphosphine and diphenylphosphine-d1. The products were measured, and the all proteo product was favored by approximately three-to-one. This measurement of product give a kinetic isotope effect of 3.1(3). This enticingly high value minimally argues for strong P–H bond activation in the turnover limiting step, but it suggests that this step is greatly impacted by specific factors of the transition state. What is enticing about this revelation is that sigma bond metathesis is a symmetry forbidden process in which the symmetry rules are relaxed by d-orbital participation. The degree of d orbital participation for tin is variable, and that these orbitals are utilized may yield new reactivity.
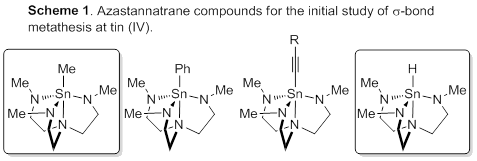
Over the past year, we have sought to develop a testable system for this hypothesis. To that end, we have returned to simple tin compounds to investigate possible sigma bond metathesis, in particular, we have been investigating derivatives in which a clear stoichiometric reaction occurs. Unlike classical transition-metal systems, Cp*2SnCl2 does not satisfy that criterion because reaction with any E–H bond does not form a single product due to multiple reaction sites and simple derivatives like Cp*2Sn(PR2)2 were not isolable in our hands. We sought to prepare azatran derivatives of tin(V) at the time of the annual report. Since then, we have prepared both the methyl and hydride compounds, which are known (Scheme 1). Interestingly, both compounds react with hydrogen gas to give either methane or hydrogen in ligand exchange processes. We have initiated the kinetic investigation of the system and data at this point is too preliminary to confirm or deny a sigma bond metathesis mechanism.
II.b. Reductive elimination
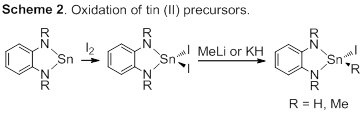
Our efforts toward identifying facile reductive elimination from Sn(IV) has been proceeding forward. As described we have been preparing selected tin(II) (i.e., stannylene) compounds that we have been oxidizing to tin(IV). As noted, many of these derivatives are more challenging to prepare than anticipated. The oxidation of these compounds was not reported, and reactions with methyl iodide or iodine resulted in the formation of the expected tin(IV) products. Precise control of stoichiometry was unexpectedly critical for both reagents. Iodide derivatives have all be converted successfully to the dimethyl derivatives, but these have not given observable formation of ethane. Hydrides have been prepared in some instances, but the purity of those compounds has been limited (Scheme 2). Clearly, pure derivatives will be needed for this work.
Unfortunately, delays resulting from our move have cost valuable time. I anticipate new publications from these efforts in the near future. Unfortunately, we lack the preliminary data to parley our initial results into a new proposal, but with persistent effort this year, I fully anticipate a federal submission next year.