Reports: DNI756820-DNI7: Synthesis of Functional Shape-Persistent Ladder Polymers
Yan Xia, PhD, Stanford University
Ladder polymers represent a unique highly rigid polymer architecture, consisting of an uninterrupted series of conformationally restrictive rings. We are interested in exploring ladder architecture as the skeleton for responsive functional polymers and expect interesting effects arising from the rigid and anisotropic ladder backbone, which is very different from commonly used flexible polymers for responsive materials.
We recently developed a new ladder polymerization method based on efficient catalytic arene-norbornene annulation (CANAL), which results in ladder polymers with contorted fused norbornyl benzocyclobutene backbones. A notable advantage of CANAL polymerization is that readily available dibromoarenes and norbornadiene (NBD) can be used as monomers. In the first step toward developing responsive functional ladder polymers, we need to evaluate the functional group compatibility of CANAL and incorporate useful functional groups into CANAL polymers.
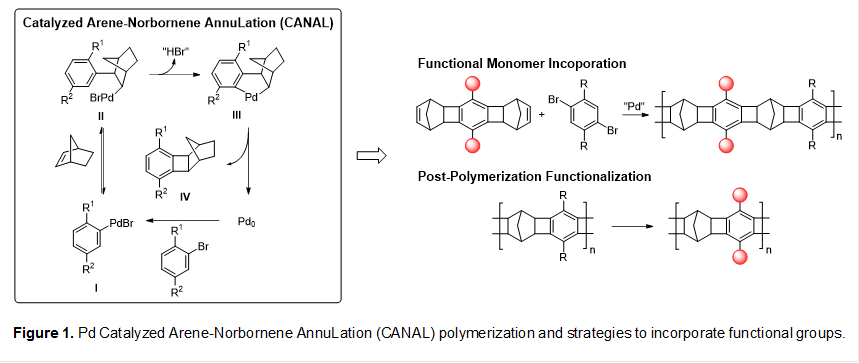
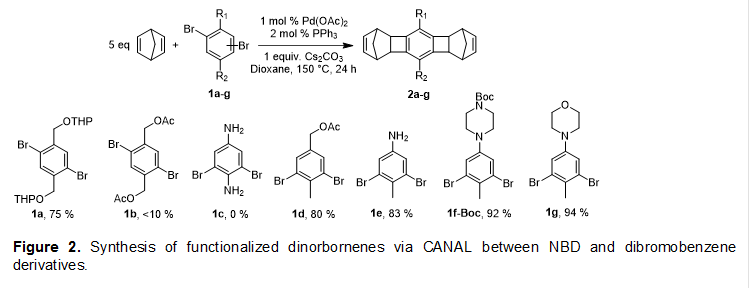
To incorporate functional groups in CANAL ladder polymers, both copolymerization of functionalized monomers and post-polymerization functionalization may be pursued. We first investigated CANAL of dibromobenzene derivatives 1a-g with excess (5-10 equiv.) NBD (Figure 2). These model reactions serve to not only assess the reaction efficiency and annulation selectivity, but also provide a new family of functionalized ladder-type dinorbornenes (diNBEs) that can be used as monomers for CANAL polymerization, olefin metathesis, and many other reactions. The reactions were carried out using 1 mol % Pd(OAc)2, 2 mol % PPh3, and 1 equiv. Cs2CO3 in 1,4-dioxane at 150 °C. Tetrahydropyran (THP) protected p-xylylene glycol (1a) gave 75% yield. The effect of potentially coordinating substituents on CANAL reactivity depends on their positions relative to the bromide. When placed ortho to the bromide as in substrates 1b and 1c, both the acetoxy and amino groups hindered the CANAL reaction. Interestingly, replacing the ortho acetoxy or amino groups with a methyl group but leaving the meta acetoxy or amino groups as in 1d and 1e restored high CANAL reactivity. Introducing saturated heterocycles, CANAL of dibromotoluene with Boc-protected piperiazine, 1i-Boc, and morpholine, 1j, gave > 90% yields.
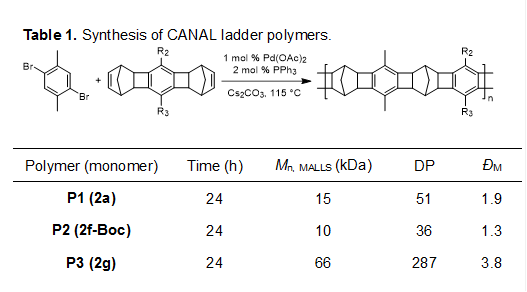
The ladder-type diNBEs underwent CANAL polymerization with dibromoxylene in complete conversions. The functionalized ladder polymers had monomodal MW distribution and relatively high MWs in the range of 10-66 kDa as measured by gel permeation chromatography (GPC) using a multi-angle laser light scattering (MALLS) detector (Mn, MALLS), corresponding to degree of polymerization (DP) of 50-300 (Table 1).
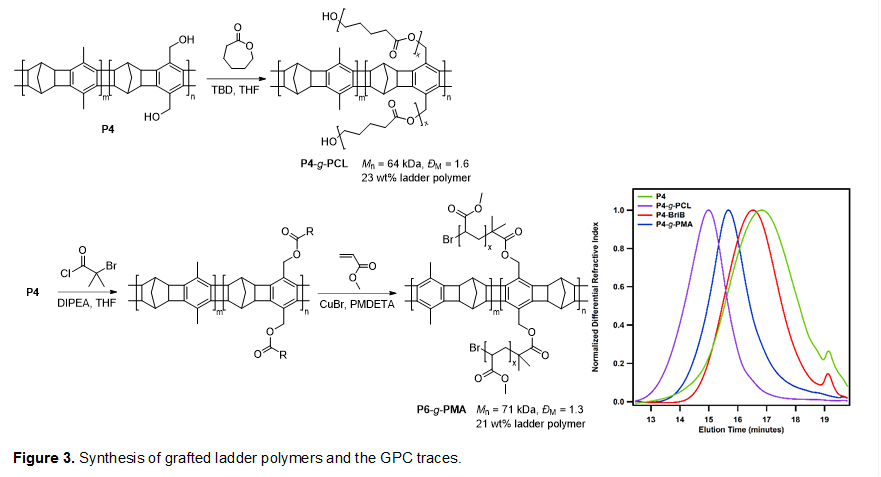
The incorporated functional groups can be further functionalized post-polymerization. In particular, we demonstrated the synthesis of sparsely grafted ladder polymers, which are interesting as they can be viewed as ladder-containing pseudo-block copolymers. Upon deprotecting the THP group on P1, The benzyl alcohol groups can be directly used to initiate ring-opening polymerization (ROP) or functionalized for other polymerizations to obtain grafted ladder polymers. We synthesized a ladder polymer P4 with 10% of the benzylic methyl positions hydroxylated. We then grafted polycaprolactone via ROP from P4 to yield P4-g-PCL. We also grafted poly(methyl acrylate) from P4 with attached initiator via atom transfer radical polymerization (ATRP) to yield P4-g-PMA (Figure 3). In both cases, GPC analysis clearly showed complete shifts of peaks from the ladder polymers to earlier elution times for the grafted polymers, while maintaining similar MWD (Figure 2). These grafted ladder polymers will facilitate the incorporation of ladder polymers into other types of polymeric materials.
This grant has provided partial support for a graduate student and a postdoctoral scholar to gain valuable training in small molecule and polymer synthesis and structural characterization. This work has been presented by the graduate student at the ACS 2017 spring meeting and lays the foundation for his thesis work on functional ladder polymers for various applications.











