Reports: DNI1055789-DNI10: Interfacial Phenomena of Versatile Janus Particles at Fluid-Adjoining Interfaces and Their Potential Applications in the Petroleum Industry
Younjin Min, PhD, University of Akron
Introduction
Janus colloidal particles with anisotropic shape and/or surface properties have attracted significant attention due to their unique morphologies, controllable molecular recognition, and self-assembling processes. Although various interesting aspects of their collective behaviors at interfaces have been studied, there are a lot of unknowns about their kinetics and dynamics of assembly into unique nanostructures and physicochemical mechanisms governing the phase behavior and stability of such nano-assemblies. This research is aimed at obtaining an understanding of interparticle interactions and interactions between a particle and the surrounding liquid for Janus nanoparticles; how these interactions govern the kinetics and thermodynamics of nanoparticulate assembly as well as phase behavior and stability of such assemblies.
Results
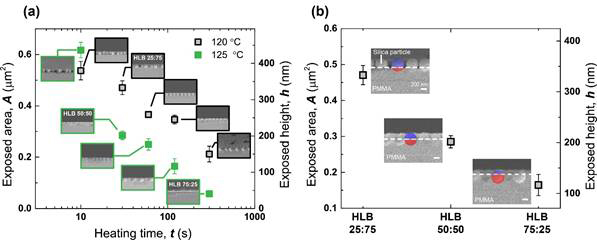
After successfully establishing solid protocols to systematically synthesizing monodisperse silica (SiO2) nanoparticles with different diameters and to generate well-defined colloidal monolayers, one of main experimental focuses during this fiscal year was to adjust Hydrophile-Lipophile Balance (HLB) ratios of silica nanoparticles in a way to control surface wetting properties at the interface between oil and water. Here, the poly(methyl methacrylate) (PMMA) thin films were first prepared in aid of flow-coating method, followed by preparation of colloidal monolayers using Langmuir-Blodgett technique. To fabricate Janus nanoparticles with three different HLB ratios of 25:75, 50:50, and 75:25, the colloidal monolayers of SiO2 (diameter ~ 440 nm) were sunk into PMMA thin films by heating PMMA above its glass transition temperature for different time scales (Figure 1).
Figure 1. (a) Exposed areas and heights of silica particles as a function of heating temperature and heating time. Insets show fractured cross-section SEM images that were taken after heat-sinking steps. The experimental conditions aiming to obtain three HLB ratios of 25:75, 50:50, and 75:25 are shown in (b). Red and blue shadows in insets of (b) represent embedded portions of silica particles into PMMA films and exposed portions of silica particles available for subsequent CVD reaction, respectively.
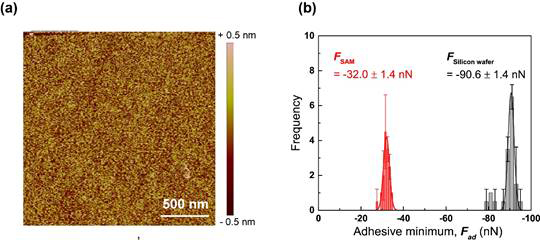
The hydrophobic modification on the exposed surface area of silica nanoparticles was carried out through chemical vapor deposition (CVD) technique using a chlorodimethylpropylsilane (C5H13ClSi). The CVD experimental conditions were first optimized using silicon wafers after transforming their surface chemistry similar to silica nanoparticles. The optimized CVD reaction yielded self-assembled monolayers (SAMs) on the silicon wafers of which thickness was estimated as about 3.4 Å with surface roughness about 0.15 nm as shown in Figure 2 (a). The force measurements in the absence and presence of SAMs were performed to further ensure the complete formation of hydrophobic layers on the surface (Figure 2 (b)). The corresponding adhesion force and interfacial adhesion energy of SAM surface were significantly decreased about three folds (about -32 nN and 340 mJ/m2, respectively), indicating that the exposed areas of silica nanoparticles were completely covered by a monolayer of chlorodimethylpropylsilane.
Figure 2. (a) AFM height image of self-assembled chlorodimethylpropylsilane layers on silicon wafer after CVD treatment. (b) Adhesion forces measured during retraction process on bare (black) and SAM covered (red) silicon wafers.
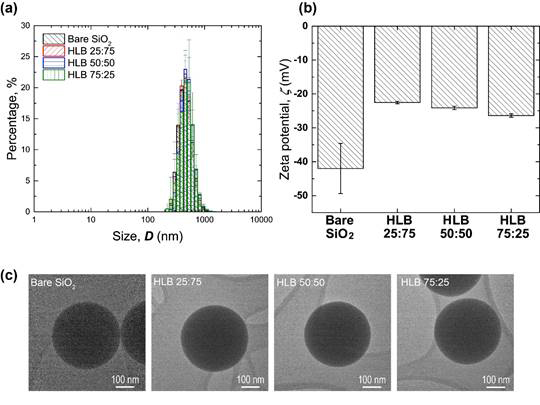
The physcochemical properties of high-quality Janus nanoparticles prepared with three different HLB ratios have been characterized by dynamic light scattering, zeta potential measurement, and transmission electron microscopy (TEM) imaging. As can be seen in Figure 3, all the hydrophobically modified Janus nanoparticles remained same in size regardless HLB ratios while the absolute surface potential values show a tendency of decreasing as the more hydrophobic surface modification was executed. The TEM images show no signs of either agglomerated chlorodimethylpropylsilane molecules on the surface or residual PMMA molecules, further confirming a successful silanization for all the Janus nanoparticles.
Figure 3. (a) Number averaged hydrodynamic sizes, (b) zeta potential values, and (c) TEM images of bare and Janus nanoparticles with different HLB ratios.
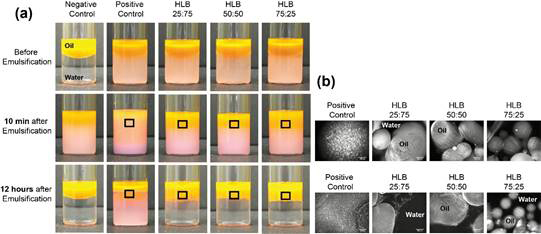
The capabilities of Janus nanoparticles as interfacial surfactants to form stable Pickering emulsions have been evaluated in a mixture of Nile-red doped tetradecane and water. Two types of controls were used in comparison; the negative control indicates oil and water mixture without any particles while the positive control contains bare SiO2 nanoparticles originally dispersed in aqueous medium. All the nanoparticles were initially placed in water phase before emulsification, displaying opaque color on the bottom (in the first raw of Figure 4 (a)). Upon emulsification in aid of ultrasonicator in pulse mode, all the samples became milky white as an indication of the formation of oil in water emulsion except negative control. The oil droplet size in positive control appears to be significantly smaller compared to the ones in emulsions stabilized by Janus nanoparticles, indicating that bare SiO2 nanoparticles are much less present at the oil-water interface. Indeed, at 12 hours after emulsification, the water phase in positive control became cloudy, demonstrating free silica nanoparticles in the aqueous phase of the sample, as similarly shown before emulsification. On the other hand, all the Janus nanoparticle stabilized Pickering emulsions appear stable up to 12 hours (continuous observation on progress). The aqueous phase of the samples containing Janus nanoparticles remain transparent as shown in the last row of Figure 4 (a), proving that most Janus nanoparticles are dedicating to stabilize the interface between oil and water of which respective Pickering emulsions are shown in Figure 4 (b).
Figure 4. (a) Photographs of oil-water mixtures in the absence (Negative Control) and presence (Positive Control: Bare SiO2, HLB 25:75, HLB 50:50, and HLB 75:25) of nanoparticles before and after emulsification at different time periods. (b) Fluorescence microscopic images of emulsions obtained from the middle region of the mixtures as shown in respective rectangles in (a). The scale bars are 200 mm.
Future work
Some remaining tasks under Objective 1, fabricating Janus silica nanoparticles with well-defined HLB ratios and Objective 2, constructing phase diagrams for water-oil-Janus nanoparticles have been covered. Our future studies will aim at obtaining dynamic time scales for the Pickering emulsion formation process. Finally, we will utilize the surface force apparatus technique to directly measure the nanoscale interactions between two oil–water interfaces containing Janus nanoparticles and interfacial coalescence behavior.