Reports: DNI755725-DNI7: Nanoemulsion-Templated Porous Polymers
Reza Foudazi, D. Tech., New Mexico State University
1. Introduction
Porous polymers have a wide range of applications in membranes, catalysts, engineered tissues, and photonic devices. While mesoporous and microporous polymers have been made using different techniques and formulations, there is a current gap of 50-200 nm range of the pore size that needs further development. A way to fill the gap is to use highly concentrated nanoemulsions as template to produce nanoporous structures in polymers. Nanoemulsions (NE) are kinetically stable form of emulsions with the internal phase size range between 50 to 500 nm. One way to increase the performance and mechanical strength of the nanoporous polymers is through formation of ordered internal phase nanoemulsion which could retain the structure after polymerization, with potential applications in water treatment membranes and photonic devices.
2. Experimental
Water-in-oil emulsion formulations consisted of styrene or ethylhexyl acrylate (monomer), divinylbenzene (crosslinker), 2,2'-azobisisobutyronitrile (AIBN, thermal initiator) and hexane (volatile organic solvent) in the continuous phase and aqueous solution of calcium chloride in the dispersed phase. The AIBN was replaced by potassium persulfate, dissolved in the dispersed phase, in some the formulations. The emulsions were prepared using ultrasonication technique with the initial aqueous phase volume fraction of 20%, stabilized by (1) polyglycerol polyricinoleate (PGPR), (2) mixtures of PGPR/Span 20, and (3) mixtures of PGPR/Tween 20. The mixing was carried on until the initial white color of the samples became translucent, indicating that the aqueous phase droplet size is in the nanometer range. Then, the emulsions volume fraction increased to 70% by removing the volatile solvent. Gradual evaporation and centrifugation approaches were used to remove the solvent and possibly order the internal phase. In the evaporation approach, the samples were kept under fume hood until the solvent was completely evaporated and then the concentrated emulsions were polymerized in the oven for 24 h at 70 oC. In the second approach, the internal phase was concentrated by applying gravitational force using ultracentrifugation at 18,000 rpm for 8 h, and then, thermally polymerized. The porous polymers were washed by isopropanol and water, then dried in a vacuum oven. The droplet size of nanoemulsions was analyzed by dynamic light scattering (DLS) via a Zetasizer (Malvern Instruments, UK). The properties of the polymerized samples were examined using BET, small angle X-ray scattering (SAXS), and transmission electron microscopy (TEM).
3. Results and Discussion
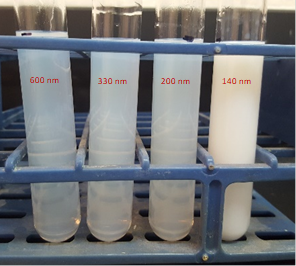
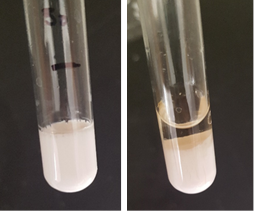
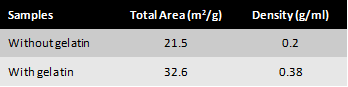
The minimum surfactant concentration of PGPR to prepare nanoemulsion is equal to the monomer+crosslinker concentration. It takes 1 h ultrasonication time to observe the transition from white to translucent color of the emulsion (Fig. 1) with d=350 nm. The minimum concentration of surfactant reduces to 10 % and mixing time to 20-40 min with the addition of Span 20 or Tween 20. The coalescence instability during evaporation is observed for all samples, although the rate of coalescence is higher for samples stabilized by PGPR/Tween 20. While the samples with droplet size higher than 300 nm are completely polymerized, phase separation occurs for samples below 300 nm size during thermal polymerization (Fig. 2).To decrease the coalescence rate, 10 wt.% gelatin was added to the aqueous phase and the samples were kept at 5°C for 2 h to induce gelation. However, the droplet size reduction below 300 nm is not possible in the presence of gelatin. The BET was performed on the two fully polymerized samples with and without gelatin in the formulation. The results showed a higher surface area of the porous polymer prepared by the addition of gelatin compared to the polymer without gelatin, despite higher density of gelatin-based sample (Table 1). Due to fragile (styrene-based) or very rubbery (acrylate-based) structures of the porous polymers, the sample preparation for TEM and SAXS is challenging and still undergoing (Fig. 3).
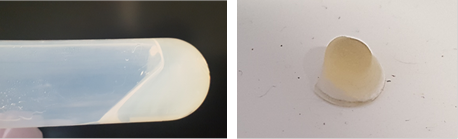
In the second approach, the samples were centrifuged for 8 h to produce concentrated NEs with a negligible degree of coalescence. However, similar to the evaporation approach instability was observed during the polymerization (Fig. 4). To reduce the instability of the NEs during polymerization, we replaced thermal initiation with the UV one (1-hydroxycyclohexyl phenyl ketone). The samples were polymerized in a UV chamber, but the instability was still observed probably due to the low intensity of the chamber.
4. Conclusion and Future work
We have produced nanoemulsions through ultrasonication which can be concentrated through evaporation and centrifugation methods. The centrifugation reduces the instability in the system, and presumably can be utilized to induce the ordering in the system. The current challenge is to polymerize NEs that remain stable during polymerization. The NEs below 150 nm are not stable during thermal and low intensity photopolymerization. We are working on a high intensity UV source set-up to cold-polymerize the samples at a shorter time.
5. The Impact of Research
The funding from ACS PRF has enabled the PI to expand his expertise to NEs. The postdoc fellow involved in this project has had the opportunity to gain hands-on experiences and skills for different characterization techniques including TEM, DLS, ultracentrifugation, and SAXS. Additionally, in an attempt to order NEs on liquid surfaces for subsequent polymerization, a side project has been defined. We could not order emulsions through spreading on liquid surfaces, but a new physical phenomena was observed, which have been reported in two Society of Rheology Annual Meetings and one article is going to be submitted in 2017.
Figure 1: From left to right is the gradual color transition from macroemulsion to nanoemulsion
Figure 2: Full polymerization of the emulsion with d= 350 nm (left) and phase separation during the thermal polymerization (right) for the emulsion d= 130 nm
Table 1: Surface area of the porous styrene-based polymers with and without gelatin in the initial emulsion formulation
Figure 3: TEM images of porous polymers with (left) and without (middle) gelatin, and a SAXS graph of the of porous polymers without gelatin (right)
Figure 4: Concentrated nanoemulsion after centrifugation (left) and after thermal polymerization (right)