Reports: UNI1056764-UNI10: Synthesis and Characterization of Protective Cr-Based Aluminides, Borides, and Carbides
J. W. Simonson, Farmingdale State College
In proposing this project, we described a course of fundamental, exploratory research into the synthesis and characterization of new Cr-based aluminides, borides, and carbides for high temperature protective applications. We targeted these materials due to: their strong covalent bonding, often resulting in high hardness; their metallic nature, typically yielding resistance to thermal shock and fracture; and their propensity for forming protective Cr2O3 and Al2O3 scales. Since then, we have compiled libraries of prospective compounds, we have grown numerous high-quality single crystals, and we have characterized structures and properties. Our work in year one focuses on three compounds: La21Cr8-2aAlyGe7-yC12, Cr55Al232-δ, and Cr3.1Ni0.93Al14.7, the first of which is entirely new, while the others have not previously been characterized. We discuss here our work on these materials, as well as the impact of this project on my career and upon the advancement of our students.
IMPACT OF THE RESEARCH
Crystal structure of La21Cr8-2aAlyGe7-yC12
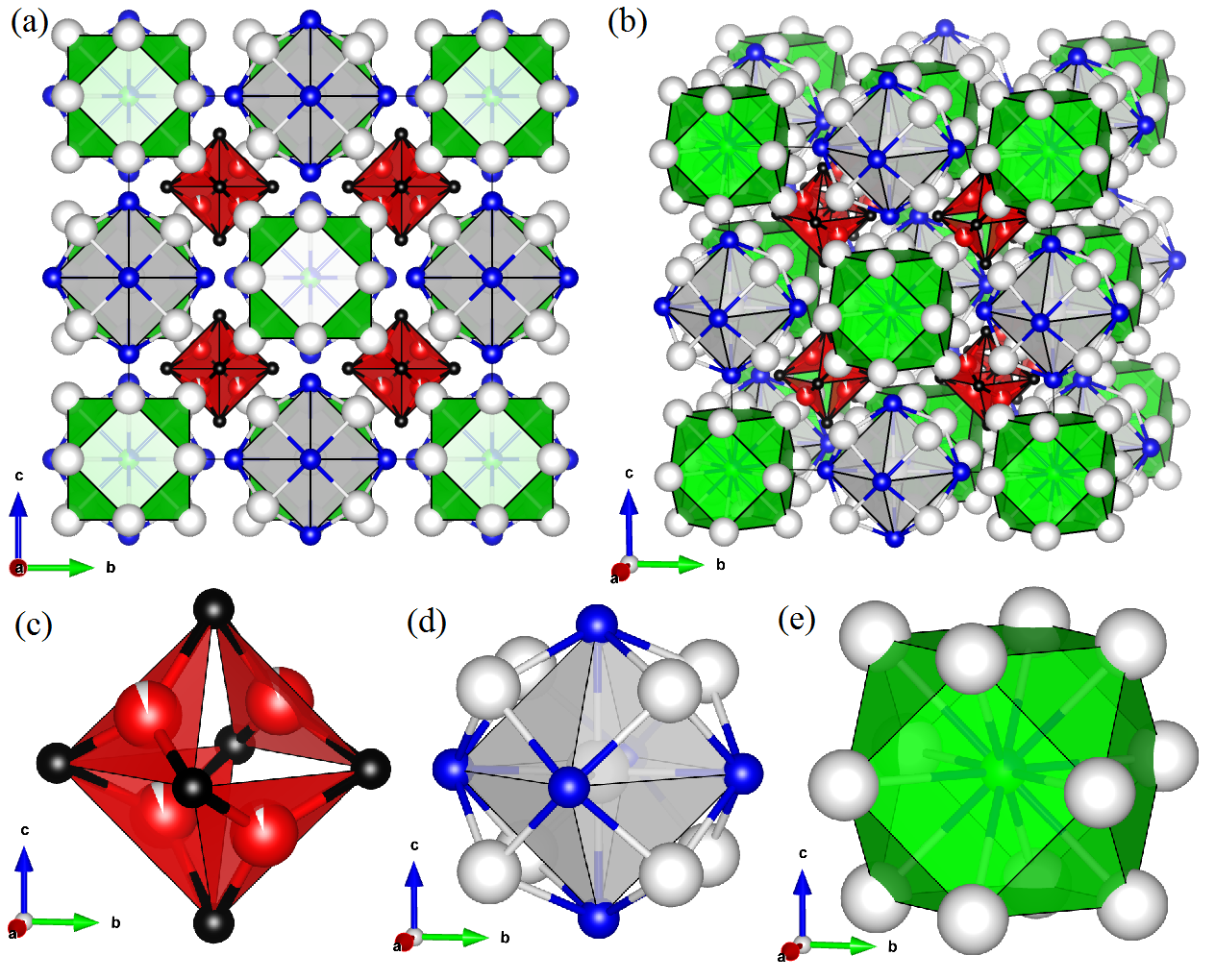
Early on, we identified La as a potential flux due to its capability to dissolve Cr, Al, and C, and our first success was growing crystals of La21Cr8-2aAlyGe7-yC12, which form as black-metallic tablets. La21Cr8-2aAlyGe7-yC12 is a new compound and the first Cr-based member of its structure type. We show in figure 1a-b the structure, which is composed of three sub-units arranged as a polyatomic analog of the Heuslers. In the figure, colors are: La (white), Cr (red), Al (green), Ge (blue), and C (black), and ellipsoids correspond to 99% probability. A La9Ge6 sub-unit (Fig. 1d) is centered on a La atom, which is octahedrally coordinated by Ge and enclosed within a cube of La. La12AlbGe1-b sub-units (Fig. 1e) consist of central Al/Ge coordinated by six equally-spaced triangular La plaquettes. Most interestingly, a sub-unit with composition Cr4-aC6 (Fig. 1c) forms four vertex-linked, tetrahedrally arranged CrC3 plaquettes. This geometrically-frustrated configuration of Cr may generate magnetic frustration and a host of magnetic ground states, as have previously been observed in pyrochlores and related compounds. Despite our interest in its magnetic properties, the high La content of this phase limits its utility at high temperatures, so we published this work and moved on to new materials.
Figure 1: Crystal structure of La21Cr8-2aAlyGe7-yC12
Hardness, oxidation resistance, and low thermal conductivity of Cr55Al232-δ
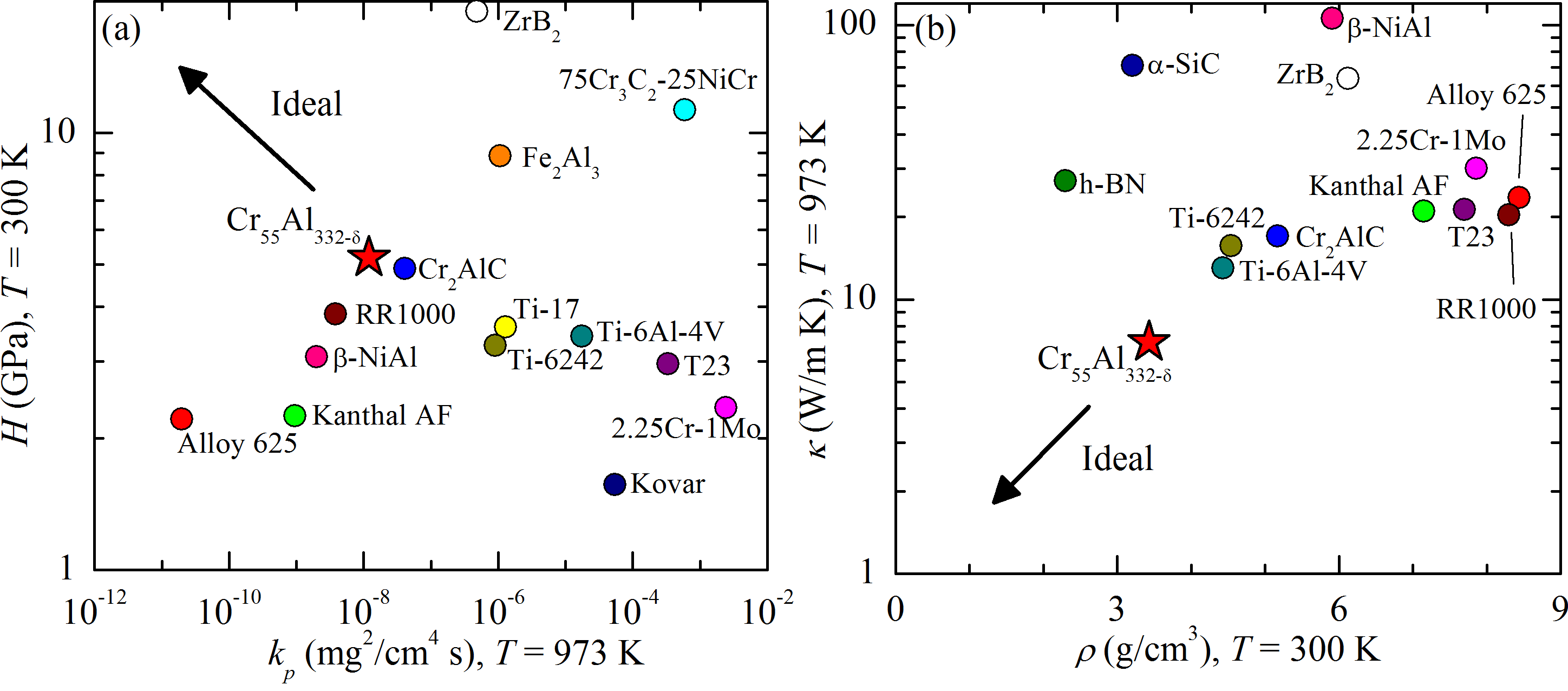
We found remarkable success in growing Al-based crystals directly from self-flux, with crystal sizes often limited only by the extent of the growth vessel. Our first foray into this space is Cr55Al232-δ, a quasi-crystalline approximant. We characterized the crystal structure, hardness, fracture toughness, oxidation resistance, oxide scale via scanning electron microscopy, high temperature thermal conductivity, and high temperature thermal expansion. We found high hardness, robust oxidation resistance, and low thermal conductivity -- all promising for the proposed application. Room temperature fracture toughness was modest, however, motivating our search for related materials. We show in figure 2 a selection of the relevant properties of Cr55Al232-δ – including hardness H, parabolic oxidative rate coefficient kp, thermal conductivity κ, and density ρ – in comparison to the properties of several high temperature alloys and refractory compounds. Among these, Cr55Al232-δ is unique in its combination of high oxidation resistance and hardness, as well as in its low density and thermal conductivity.
We submitted a manuscript on the favorable properties of this material, and we are presently addressing referee comments. We expect to resubmit in the coming weeks.
Figure 2: Contextual plot of the properties of Cr55Al232-δ
Composition, low thermal conductivity, and high hardness of Cr3.1Ni0.93Al14.7
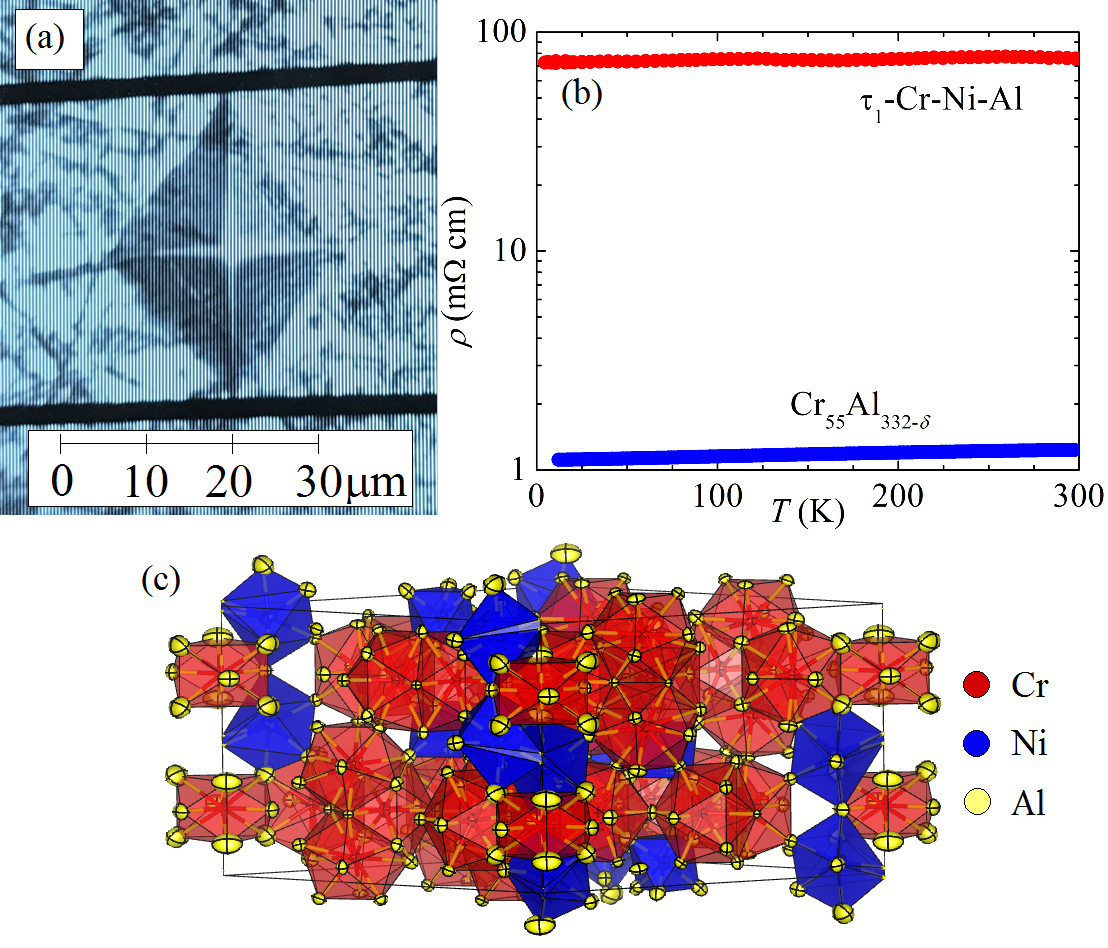
Cr3.1Ni0.93Al14.7 is our second generation material, in which we seek to improve upon the strengths of Cr55Al232-δ while addressing its limitations. We clarify a discrepancy in the literature regarding the composition and space group this material. As we show in Fig. 3a, Vickers micro-hardness indentations reveal striking improvement over Cr55Al232-δ with H=6.88(13) GPa and a lack of visible indentation fractures, speaking to substantially improved fracture toughness, which we are preparing to quantify. Fig. 3b shows that electronic transport in this compound is diminished by two orders of magnitude from Cr55Al232-δ, suggesting that thermal conductivity will likewise be reduced.
We ascribe these improvements to intrinsic structural disorder, our model of which is shown in Fig. 3c. Some uncertainties remain, and we have received neutron diffraction time at the NIST Center for Neutron Research to clarify the relative occupancies of Cr and Ni on the transition metal sites.
Figure 3: Properties and structure of Cr3.1Ni0.93Al14.7
IMPACT ON THE PI
I am a strong advocate for applied and experiential learning, such as the ACS-PRF has made possible through this project. This funding has permitted the acquisition of consumable supplies for our research. It has allowed me to remain research-active at a teaching-focused undergraduate institution. It has permitted me to travel to conferences to share the results of our work. Perhaps most importantly, the research products we create through this project will form the bedrock for further proposals for external funding as my career continues.
IMPACT ON THE STUDENTS
Over the first year of this project, we supported seven undergraduate students -- more than double the number we had originally anticipated. Two of these students are from populations historically underrepresented in STEM fields and received additional support the New York State Collegiate Science Technology Enrichment Program CSTEP. Five of the seven students are economically disadvantaged and receive substantial financial aid that permits them to attend college. The stipends these students received from this project permitted them to work on campus in a laboratory related to their intended career paths rather than work off campus in unrelated retail jobs.
This project generated six student poster and oral presentations and local, state, and national scientific meetings including the Farmingdale State College Celebration of Scholarship, the SUNY Undergraduate Research Conference, the NYS CSTEP Conference, and the Emerging Researchers National Conference.
Our first senior student member of the project graduated in May 2017 and is now pursuing an M.S. in civil engineering at Manhattan College. Another student member used the experience of being part of the project to transfer to Yale University. Three senior students are presently preparing applications to PhD. programs.