Reports: UR354825-UR3: Phosphine-Directed C-H Borylation of Arenes: Facile Access to Ambiphilic Phosphine Boronate Esters
Timothy B. Clark, PhD, University of San Diego
Progress Report
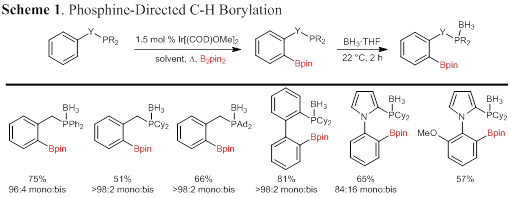
Just prior to the start of this grant, we reported the phosphine-directed C–H borylation reaction shown in Scheme 1. To the best of our knowledge this transformation represents the first example of a phosphine-directed C–H functionalization reaction (Scheme 1). This early set of reaction conditions provided a series of phosphine boronates that were purified upon protection of the electron-rich phosphines as the borane complex. The scope of the reaction was somewhat limited however, and more general reaction conditions were required to access a large array of phosphine boronate esters for applications as ambiphilic (compounds with Lewis acid and base) catalysts and ligands.
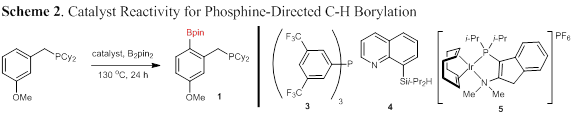
The initial focus of this grant was to identify new catalytic conditions that were amenable to a wider range of phosphine substrates. 3-Methoxybenzyldicyclohexylphosphine (1, Scheme 2) was chosen as the model substrate for optimization based on its complete lack of reactivity under the previously reported reaction conditions. Several catalyst systems used for other directing groups were examined but none of them were found to mediate the desired reaction. Cationic pre-catalyst 5 was examined with the thought that the cationic nature of the complex would replace one of the X-type Bpin ligands with a non-coordinating ligand, opening a coordination site for C–H activation. Under these reaction conditions, a modest conversion of 33% was observed (entry 5).
Entry |
Catalyst |
Ligand |
Conv. |
1 |
[(COD)IrOMe]2 |
none |
NR |
2 |
[(COD)IrOMe]2 |
2 |
NR |
3 |
[(COD)IrOMe]2 |
3 |
NR |
4 |
[(COD)IrOMe]2 |
4 |
NR |
5 |
[Ir(P,N)]PF6 (5) |
none |
33% |
Boron (equiv) |
Temp (oC) |
t (h) |
Conv. |
Select. |
B2pin2 (0.83) |
130 |
24 |
0% |
>99:1 |
HBpin (1.7) |
130 |
24 |
72% |
81:19 |
HBpin (6.7) |
130 |
24 |
99% |
87:13 |
HBpin (3.3) |
110 |
18 |
99% |
>99:1 |
The unusual reactivity of cationic complex 5 with phosphine 1 led us to screen other cationic iridium complexes with rigid, electron-rich bidentate ligands that are commonly used for non-directed C–H borylation. The combination of [(COD)2Ir]BF4 and TMPHEN was found to be unreactive with B2pin2 (Scheme 2, right), but highly reactive with pinacolborane (HBpin). The equivalents of HBpin used and the reaction temperature and time was optimized, allowing the formation of phosphine boronate 1 to occur after 18 h at 110 °C.
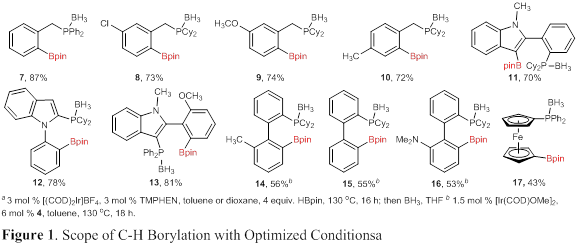
Multiple phosphines have been examined under these optimized reaction conditions, providing a variety of phosphine boronate esters in moderate to good isolated yield upon protection as the borane complex (Figure 1). Borylated benzylic phosphines were isolated in consistently high yield (7–10). Indole-based phosphines were equally reactive, providing 11–13 in high yield. Biphenylphosphines were not as reactive under the optimized reaction conditions, but were found reactive when silylquinoline (4, Scheme 2) was used as the ligand. Finally, a ferrocenyl-based phosphine was reactive under the optimized conditions to provide 17 in moderate yield. The decreased yield in this case is due to competitive bis-borylation.
 |
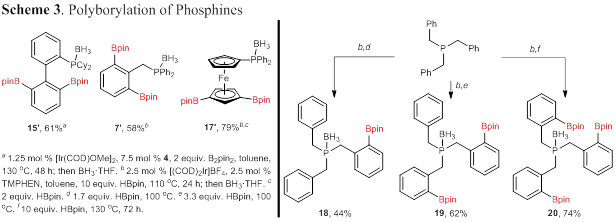
The observance of bis-borylation products for some substrates led us to examine the ability to selectively access polyborylation products by modifying the reaction temperature and equivalents of the boron source. Bis-borylation products 15′, 7′, and 17′ were isolated in moderate to high yield (Scheme 3). Reaction of tribenzylphosphine, under a series of conditions, allowed the isolation of mono-, bis-, and tris-borylated products in moderate to good yield. Polyborylated phosphines are envisioned to be useful in understanding the role of the Lewis acidic boron when examining these ambiphilic compounds as organocatalysts and bifunctional ligands (a future direction).
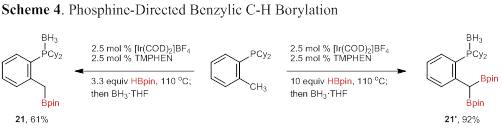
The final area of phosphine-directed C–H borylation we have examined is of benzylic C–H bonds. Substrates with an adjacent benzylic methyl provided a complex mixture of borylation products. Recognizing that some of these products likely result from benzylic C–H borylation, a substrate that lacked accessible aryl C–H bonds was chosen. Dicyclohexyl(2- methylphenyl) phosphine was subjected to the standard reaction conditions: 3.3 equivalents of pinacolborane provided monoborylation product 21 in 61% yield; a 92% yield of 21′ was obtained with 10 equivalents of pinacolborane (Scheme 4).
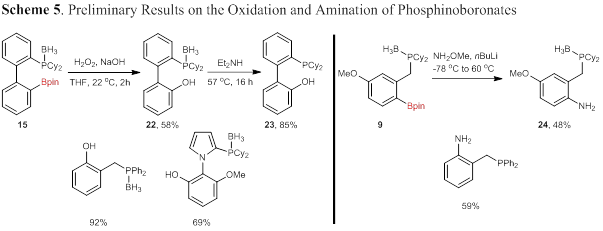
Reactions that utilize the carbon–boron bond for functionalization to access bifunctional phosphines was initiated. Oxidation to form phenols has been achieved by our lab with several protected phosphine boronates with no evidence of deprotection under the reaction conditions (Scheme 5, left). Importantly, the resulting phenol (22) is readily deprotected to form the corresponding phosphinophenol 23 in high yield. Phosphinophenols have received considerable attention as bidentate ligands but are generally limited to ortho-phosphinophenols. Additional phosphine boronates will be utilized in oxidation reactions, broadening the scope of phosphinophenols, such as 23, that are readily accessible could have a significant impact on reactions utilizing these ligands. Amination of protected phosphine boronate 9 using conditions developed by Morken132 has also provided the corresponding aminophosphine (24) in moderate yield. Diphenylphosphine 7 was found to result in deprotection of the phosphine under the reactions conditions, likely the result of a less electron-rich phosphine. In both cases, further optimization is required to improve the yields and to demonstrate the generality of the method.
Impact of Award
This ACS-PRF award has catalyzed this project into a robust research project in the PI’s lab. A grant to the National Science Foundation (Research at Undergraduate Institutions) was recently submitted based on the work funded by this grant. We are currently writing up the work described above for submission to the Journal of the American Chemical Society. During the last year, this grant has supported three different students. Two of those students have graduated. One is currently spending a year with Teach for America with the goal of becoming a High School chemistry teacher. The second has started as a graduate student at Colorado State University. The third student has just started her junior year at USD and has matured significantly over her first semester and summer doing research in the PI’s laboratory.